Would you like to make this site your homepage? It's fast and easy...
Yes, Please make this my home page!
HOME~~~PREV~~~NEXT
~~~ CELLULAR PHYSIOLOGIC CONTROL MECHANISMS ~~~
1. GROWTH FACTORS
2. CYTOKINES
3. CYCLIC AMP or GMP
4. CALMODULIN
5. NITRIC OXIDE
6. DOCOSOIDS
7. PROTEIN PHOSPHORYLATION
8. NUCLEAR BINDING OF STEROIDS
9. *** REDOX STATUS ***
~~~ ANTIOXIDANT ADAPTATION ~~~
Prooxidant conditions trigger the induction
of various antioxidant enzymes such as:
- glutathione reductase
- glutathione synthase
- glutathione peroxidase
- superoxide dismutase
- catalase
Temporary prooxidant conditions if not beyond
the organism's ability to resist and adapt will:
- redistribute reducing equivalents
passively and actively
- enhance resistance to oxyradical damage
- inhibit pathology dependent syntheses
~~~ ENZYMATIC OXYRADICAL QUENCHING ~~~
Superoxide Dismutase:
-OO* + Enz (Cu++) --> O2 + Enz (Cu+)
-OO* + Enz (Cu+) + 2 H+ --> H2O2 + Enz(Cu++)
Catalase:
2H2O2 + Enz (Fe) --> O2 + H2O + Enz (Fe)
Glutathione Peroxidase:
2 GSH + HOOH --> GSSG + 2 HOH
2 GSH + LOOH --> GSSG + LOH + HOH
~~~ REDOX CONTROL OVER OXY-HEMOGLOBIN DISSOCIATION ~~~
Reduced glutathione (GSH) is converted to the
disulfide (GSSG) by the action of oxyradicals
and by glutathione peroxidase.
The presence of elevated levels of GSSG triggers a
change in glucose metabolism in an effort to shift
the utilization of hydrogen towards GSSG reduction.
This change results indirectly in higher levels
of 2,3-diphosphoglycerate (2,3-DPG).
2,3-DPG attaches to oxyhemoglobin in such a way as to
enhance dissociation or release of diatomic oxygen.
Thus mildly pro-oxidant conditions enhances
the delivery of diatomic oxygen to tissues.
~~~ OXIDATIVE RESCUE OF CYTOCHROME A ~~~
To review a list of known toxic agents produced
by the putrifying action of colonic anaerobes,
one of the most toxic is hydrogen sulfide (H2S).
Similar to cyanide (CN-), carbon monoxide (CO), and
azide (NN-) the bisulfide anion (HS-) attaches
to the copper of cytochrome a3 as a ligand.
This shuts down the action of an entire respiratory
electron transport chain.
This also results in failure to phosphorylate ADP,
plus a rapid and profound accumulation of reductants.
Pro-oxidant conditions, however, can increase
the RSSR' / RSH ratio.
All disulfides readily absorb hydrogen sulfide.
R'SSR" + HSH ---> R'SSH + HSR"
Thus a hypothetical mechanism of H2S detoxification,
rescue of mitochondrial phosphorylation,
and restoration of normal redox potentials
should result from treatment with oxidants.
~~~ REDOX CONTROL OVER LEUKOTRIENE SYNTHESIS ~~~
Leukotrienes are long acting promotors of inflammation.
Many of these are produced by combining glutathione
with certain proinflammatory docosoids.
Oxidation of reduced glutathione hypothetically
inhibits this process.
This opens the possibility for mildly oxidizing conditions
to have significant antiinflammatory effects.
~~~ MECHANISM OF GLYOXALASE ~~~
Reduced glutathione acting as a nucleophile spontaneously
adds to methylglyoxal producing a thiohemiacetal.
O O OH O
G-S-H + HC--C--CH3 --> G-S-C--C--CH3
H
Glyoxalase I tautomerizes the thiohemiacetyl as shown.
OH O O OH
G-S-C--C--CH3 --> G-S-C--C--CH3
H H
Glyoxalase II hydrolyses the alpha-hydroxy-acyl product
of glyoxalase I yeilding reduced glutathione and lactic acid.
O OH O OH
G-S-C--C--CH3 + HOH --> G-S-H + H0-C--C--CH3
H H
~~~ REDOX CONROL OF GLYOXALASE ~~~
Methylglyoxal was discovered by Albert Szent-Gyorgyi,
et al, to be a physiologic inhibitor of cell division.
Its level is controlled by the enzyme "glyoxalase",
which converts methylglyoxal to lactic acid.
However, glyoxalase requires reduced glutathione
(GSH) as a cofactor.
Oxidation of glutathione inhibits the action
of glyoxalase.
As the levels of methylglyoxal rise proliferation
is inhibited.
~~~ REDOX CONTROL OF ENZYMATIC FUNCTION
BY THIOL / DISULFIDE CONVERSION ~~~
The ease of conversion of thiols to disulfides by oxidation,
RSH + R'SH + [O] ---> RSSR' + H2[O]
And the ease of reversion of disulfides
back to thiols by reduction,
RSSR' + 2[H] ---> RSH + R'SH
Provide an important mechanism for physiologic control.
Many enzymes which depend upon a cysteine residue
at the active center or as part of the structure
can be switched to the [OFF] mode by oxidation.
This may result in an autogenous disulfide
or the attachment of an exogenous sulfide.

~~~ ORNITHINE DECARBOXYLASE ~~~
Ornithine decarboxylase (ODC) is an enzyme,
which is induced in all proliferating cells.
ODC converts ornithine (an amino acid) to putrescine,
an essential intermediary in polyamine synthesis.
NH2--CH2--CH2--CH2--CH2--NH2
ODC has a cysteine residue, necessary to its function,
which is maintained in the reduced state by other thiols.
The thiol group of ODC is sensitive to oxidation,
and such oxidation inactivates this enzyme.
Prooxidant conditions therefore temporarily inhibit ODC
activity and polyamine dependent cell proliferation.
~~~ REDOX CONTROL OF POLYAMINE FUNCTION ~~~
REDUCTION FAVORS:
Ornithine Decarboxylase Activation
Polyamine Synthesis
Polyamine Preservation
Growth And Proliferation
OXIDATION FAVORS:
Ornithine Decarboxylase Deactivation
Inhibition Of Polyamine Synthesis
Oxidative Conversion To Aldehydes
Inhibition Of Growth And Proliferation
"REDOX SIGNALING AND THE CONTROL OF CELL GROWTH AND DEATH"
Advances In Pharmacology [series],
Volume 38, pp 329-359, Academic Press, 1997
by Garth Powis, John Gasdaska, Amanda Baker
at Arizona Cancer Center, University of Arizona, Tucson
CONTENTS:
I. oxidation-reduction as a physiologic control mechanism
II. cellular redox systems: glutathione, thioredoxin,
protein disulfide isomerase, glutaredoxin,
Ref-1, metallothionein
III.redox targets: ribonucleotide reductase,
receptor proteins, transcription factors:
OxyR, NFkB, AP-1, p53, kU, v-Ets, E2, Ah,
protein folding & degradation
IV. cellular responses to redox changes:
proliferation, thioredoxin activity,
oxidant signaling and apoptosis,
oxidative stress, hypoxia
Bibliography contains 204 references.
~~~ REDOX ACTIVE PROTEIN THIOLS ~~~
"Thioredoxin" (Trx) is a low molecular weight protein
baring two closely situated cysteine residues,
which offer two redox active thiol groups.
In vivo Trx redox cycles between its reduced dithiol
form Trx-(SH)2 and its oxidized disulfide form Trx-SS.
Trx-(SH)2 + [O] ---> Trx-SS + H[O]H
Trx-SS + 2[H] ---> Trx-(SH)2
The flavoprotein "thioredoxin reductase" utilizes
reducing equivalents from NADPH to maintain Trx
in the reduced state.
NADPH ... FAD / FADH2 ... Trx-SS / Trx-(SH)2
Protein thiols such as Trx, protein disulfide isomerase
(PDI), and Ref-1 are important intracellular carriers
of hydrogen atoms.
These in turn maintain various other proteins in the
reduced state, and maintain certain oxidant sensitive
enzymes, growth factors, and transcription factors
in the active state.
Activator-Protein-1 (AP-1) is a transcription factor
and is activated by thiol proteins as follows:
NADPH ... FAD/FADH2 ... Trx-SS/Trx-(SH)2 ...
... Ref-1-SS/Ref-1-(SH)2 ... AP-1-SS/AP-1-(SH)2
Reduced Trx also serves to convert
ribonucleoside-5'-diphosphates to
deoxyribonucleoside-5'-diphosphates.
RNPP-OH + Trx-(SH)2 ---> dRNPP-H + Trx-SS + H2O
The redox sequence is as follows:
NADPH ... FAD/FADH2 ... Trx-SS/Trx-(SH)2 ...
RNPP-OH/dRNPP-H
Glutaredoxin (Grx) performs the same role as thioredoxin
(Trx), however, Grx must accept hydrogen from
glutathione (GSH).
NADPH ... FAD/FADH2 ... GSSG/GSH ...
Grx-SS/Grx-(SH)2 ... RNPP-OH/dRNPP-H
Thus reductants as supplied by NADPH and carried
by thiol proteins are necessary for a variety
of proliferation related functions.
~~~ TRIGGERING OF APOPTOSIS ~~~
Apoptosis (programmed cell death) is a normal
physiologic process whereby certain cells
are selected for self-destruction.
This may occur as a part of embroyologic
developement or as a mechanism to remove aged,
damaged, or malfunctioning cells.
A beneficial result would be the termination
of IgE producing plasma cells or tumor cells.
One of the physiologic triggers of apoptosis
is an oxidant sensing mechanism.
When successful such apoptosis can produce long
term beneficial results from oxidative therapies.
~~~ P53 PROTECTOR OR ONCOGENE? ~~~
P53 stands for the genome and corresponding
protein involved in several control systems:
-- detection of DNA damage
-- initiation of DNA repair
-- promotion versus suppression of proliferation
-- initiation of apoptosis by oxidants, etc.
Healthy P53 protein has cysteine residues,
which are sensitive to oxidation, making
redox control part of normal P53 function.
Mutation(s) of the P53 genome in favor
of carcinogenesis result in:
-- promotion function locked
-- apoptosis function locked
-- sensitivity to oxidants decreased or absent
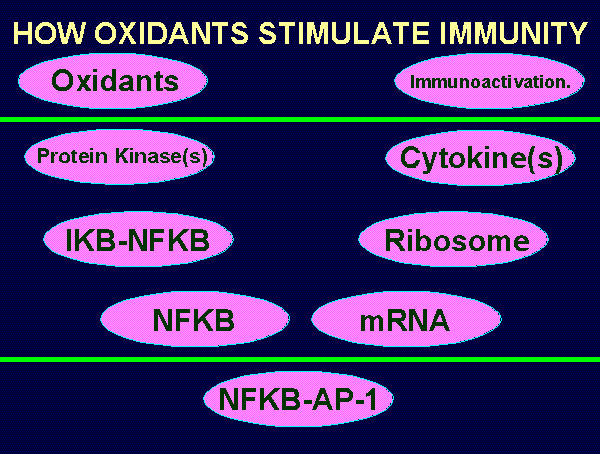
~~~ REDOX CONTROL OVER IMMUNOLOGIC TRIGGER MECHANISM ~~~
Most redox sensitive enzymes and transcription factors are
inhibited upon oxidation and reactivated upon reduction.
Conversely, certain other enzymes (namely protein kinases)
are activated upon oxidation and inhibited upon reduction.
A wide variety oxidants (including ultraviolet light
and GSSG), can activate specialized protein kinases
in immune cells.
Immune cells have protein complexes composed of
"inhibitor kappa B" and "nuclear factor kappa B".
Activated protein kinases phosphorylate I-kappa-B.
Ubiquitin attaches to phosphorylated proteins.
The proteosome subsequently eliminates I-kappa-B
by digestion.
NF-kappa-B is released and migrates to the nucleus.
There it associates with the transcription protein AP-1.
Messenger RNA is synthesized which in turn
directs the ribosomes to produce cytokines.
Cytokines function as alarms which trigger activation
of systemic immunity.
~~~ GSSG AS COFACTOR IN IMMUNOACTIVATION ~~~
In many types of cells oxidants can directly
activate protein kinases without involving GSSG.
However, in certain immune cells GSSG is a
necessary cofactor to protein kinase activation.
In these cells, deficiency of GSH and/or GSSG
results in failure of NFKB transactivation.
This explains how glutathione deficiency is
associated with immune dysregulation.
~~~ INHIBITION OF HISTAMINE RELEASE ~~~
Histamine is a proinflammatory agent produced
by the enzymatic decarboxylation of histidine.
Histamine is stored in granules within mast cells.
Mast cells degranulate and release histamine
upon action of IgE-antigen complexes.
The release of histamine from mast cells is inhibited
by certain enediols such as: ascorbic acid,
quercetin, and other polyphenolic bioflavonoids.
Which phase (oxidized or reduced) of the enediol
compound is actually responsible for this
inhibition remains to be clarified.
~~~ REVIEW OF PHYSIOLOGIC EFFECTS OF OXIDANT EXPOSURE ~~~
1. Passive and active redistribution of reducing
equivalents towards oxidant eliminating pathways.
2. Adaptive induction of oxyradical quenching enzymes.
3. Enhanced oxyhemoglobin dissociation.
4. Temporary detoxification of hydrogen sulfide.
5. Inhibition of leukotriene synthesis.
6. Inhibition of glyoxalase.
7. Reversible inhibition of numerous enzymes
and physiologic triggers which utilize thiols
in their active centers.
8. Initiation of apoptosis in unwanted cells.
9. Induction of cytokine expression.
10. Modulation of histamine release.
HOME~~~PREV~~~NEXT

