Would you like to make this site your homepage? It's fast and easy...
Yes, Please make this my home page!
HOME~~~PREV~~~NEXT
~~~ PURPOSES OF BIOLOGICAL ELECTRON TRANSFER SEQUENCES ~~~
QUESTION: Why are BETS so important?
ANSWERS: They provide the following services:
synthesis
energy production
oxidative removal of unwanted reductants
elimination of certain oxidants
xenobiotic metabolism
physiologic regulation
Failure to supply adequate reducing equivalents
can result in:
synthetic failure, fatigue,
oxyradical damage, dysregulation
Failure to remove reducing equivalents
favors diseases of:
toxicity, allergy, infection, cancer
~~~ REDUCTANT ACTIVATION SYSTEMS ~~~
These enzyme systems release electrons
and hydrogen atoms from food molecules.
In the process NAD+ and NADP+ are reduced
to become NADH and NADPH respectively.
1. GLYCOLYSIS:
D-glyceraldehyde-3-phosphate ... NAD+/NADH
2. HEXOSE MONOPHOSPHATE SHUNT:
Glucose-6-phosphate ... NADP+/NADPH
6-Phosphogluconate ... NADP+/NADPH
3. TRICARBOXYLIC ACID CYCLE:
Pyruvate...Lipoamide-SS/LA-(SH)2...FAD/FADH2...NAD+/NADH
Isocitrate ... NAD+/NADH
Alphaketoglutarate...LA-SS/LA-(SH)2...FAD/FADH2...NAD+/NADH
Malate ... NAD+/NADH
4. AMINO ACID DEGRADATION:
L-glutamate ... NAD+/NADH
L-glycine ... NAD+/NADH
5. FATTY ACID DEGRADATION:
Acyl-CoA ... NAD+/NADH
L-3-hydroxyacyl-CoA ... NAD+/NADH
6. ALCOHOL DEGRADATION:
Et-OH ... NAD+/NADH
7. PYRIDINE INTERCHANGE:
NAD+/NADH ... NADP+/NADPH ... NAD+/NADH
~~~ BETS WHICH FEED SYNTHETIC PATHWAYS ~~~
Note the dependence of numerous synthetic functions
on reducing equivalents supplied by NADPH.
1. FOLATE REDUCTASE (tetrahydrofolate synthesis):
NADPH ... FH2/FH4
2. BIOPTERIN (tyrosine synthesis):
NADPH ... BH2/BH4 ... Phe+O2/Tyr+H2O
3. THIOREDOXIN and GLUTAREDOXIN
(deoxyribonucleoside synthesis):
NADPH...FAD/FADH2...Trx-SS/Trx-(SH)2...RNPP-OH/dRNPP-H
NADPH...FAD/FADH2...GSSG/GSH...
Grx-SS/Grx-(SH)2 ... RNPP-OH/dRNPP-H
4. ORNITHINE DECARBOXYLASE (polyamine synthesis):
NADPH ... FAD/FADH2 ... GSSG/GSH ... ODC-SS/ODC-SH
5. ACP-REDUCTASES (fatty acid synthesis):
NADPH ... beta-ketoacyl-S-ACP/beta-hydroxyacyl-S-ACP
NADPH ... crotonyl-S-ACP/enoyl-S-ACP
6. HYDROXYMETHYLGLUTARYL-COENZYME-A-REDUCTASE
(turpene synthesis):
NADPH ... HMG-CoA/Mevalonic Acid
7. GLUTAMATE DEHYDROGENASES (glutamate synthesis):
NADPH or NADH ... aKG+NH3/Glu
8. ASPARTATE SEMIALDEHYDE DEHYDROGENASE
(threonin synthesis)
NADPH...beta-aspartyl-phosphate/aspartic-beta-semialdehyde
9. OTHER REDUCTASES (synthesis of other amino acids)
(steroid production / conversion)
~~~ BETS USED IN OXIDATIVE PHOSPHORYLATION ~~~
The mitochondria convey reductants to oxygen to produce water.
The enzymes involved in this BETS conserve energy by pumping
protons (H3O+) out of the inner mitochondrial space.
The energy of this acid gradient is used to convert ADP to ATP.
The BETS chiefly involved in oxidative phosphorylation
is summarized below:
NADH ... Fe2S2 ... FMN/FMNH2 ... Fe2S2 ... CoQ/CoQH2 ...
cytB-Fe+++/cytB-Fe++ ... cytC-Fe+++/cytC-Fe++ ...
cytA-Fe+++&Cu++/cytA-Fe++&Cu+ ... OO/HOH
Most of the NADH used to fuel oxidative phosphorylation
is generated within the mitochondria by the Kreb's cycle.

~~~ MECHANISM OF ISCHEMIA REPERFUSION INJURY ~~~
Under aerobic conditions electrons pass
among various carriers in a sequence.
They are ultimately combined with H+ and O2
to produce water.
4e- + 4H+ + O2 ---> 2 H2O
Elevated ADP levels trigger more electrons
to be released into this sequence.
These are readily taken up by an abundance of oxygen.
Minimal quantities of electrons leak
out of this system combining directly
with oxygen to produce superoxide.
e- + O2 ---> *OO-
During ischemia low ATP & high ADP levels stimulate
production of excessive levels of reducing
equivalents which saturate carriers.
Upon reintroduction of O2, many electrons transfer
directly to O2 producing -OO* in toxic quantities.

~~~ CYTOSOL <--> MITOCHONDRIA SHUTTLES ~~~
The inner space of the mitochondria is separated
from the cytosol by two membranes.
Thus neither NADH nor NADPH from the cytosol
can enter the mitochondria.
Instead BETS known as shuttles exist.
These transfer reducing equivalents between
the cytosol and the mitochondria
(where they are consumed in ATP production).
THIOCTIC (ALPHA-LIPOIC) ACID SHUTTLE:
TA-SS- / TA(SH)2 ... NAD+/NADH ... FAD/FADH2 ...
CoQ/CoQH2 ... cytochromes ... OO/HOH
GLYCEROL-3-PHOSPHATE SHUTTLE:
NADPH ... DHAP/G3P ... FAD/FADH2 ...
CoQ/CoQH2 ... cytochromes ... OO/HOH
MALATE SHUTTLE:
NADH ... OAA/Malate ... NAD+/NADH ... FAD/FADH2 ...
CoQ/CoQH2 ... cytochromes ... OO/HOH
ISOCITRATE SHUTTLE:
OSA/ICA ... NADP+/NDAPH ... FAD/FADH2 ...
CoQ/CoQH2 ... cytochromes ... OO/HOH
NAPHTHOQUINONE (such as menadione) SHUTTLES:
NADPH ... FAD/FADH2 ... GSSG/GSH ... NQ/NQH2
... cytochromes ... OO/HOH
~~~ CYTOSOL <--> MITOCHONDRIA SHUTTLES ~~~
THIOCTIC ACID DITHIOL: THIOCTIC ACID DISULFIDE:
H H H H H H H H
HC---C---C---(C)4---C=O HC---C---C---(C)4---C=O
| H | H | | H | H |
SH SH OH S-------S OH
GLYCEROL-3-PHOSPHATE: DIHYDROXYACETONE-PHOSPHATE:
H H H H H
HC---C---CH HC---C---CH
| | | OH | || | OH
OH OH O--P=O OH O O--P=O
OH OH
MALIC ACID: OXALOACETIC ACID:
H H H
O=C---C---C---C=O O=C---C---C---C=O
| | H | | || H |
OH OH OH OH O OH
ISOCITRIC ACID: OXALOSUCCINIC ACID:
H H H H H
O=C---C---C---C---C=O O=C---C---C---C---C=O
| | | H | | || | H |
OH OH COOH OH OH O COOH OH
MENADIONOL: MENADIONE:
HC--C--CH3 HC==C--CH3
// \\ / \
HO-C C-OH O=C C=O
\ / \ /
RING RING
~~~ OXIDANT ELIMINATION PATHWAYS FED BY NADPH & GSH ~~~
Glutathione (GSH) is the main hydrogen carrier
feeding various oxidant elimination systems.
GSH is kept predominantly in the reduced phase
by glutathione reductase.
NADPH ... FAD/FADH2 ... GSSG/GSH
GSH maintains many cytosolic thiols in the reduced state.
GSSG/GSH ... RSSR'/RSH+HSR'
GSH reactivates various reductive antioxidants.
GSH...CoQ/CoQH2...vitE-O*/vitE-OH...ROO*/ROOH
GSH...CoQ/CoQH2...Dehydroascorbate/Ascorbate...HOO*/HOOH
GSH...CoQ/CoQH2...Tannin/Polyphenol...HO*/HOH
GSH activates glutathione peroxidase.
GSH ... Se/SeH ... ROOH/ROH+HOH
GSH ... Se/SeH ... HOOH/HOH+HOH
~~~ GLUTATHIONE PEROXIDASE SYSTEM ~~~
This is a biological electron transfer sequence.
Under usual circumstances sugar phosphates
as occur in the hexose monphosphate shunt
serve as initial hydrogen donors.
Hydrogen atoms can also be supplied by other
enzymatic systems of NADP+ activation.
The final acceptors of hydrogen are:
hydrogen peroxide (HOOH)
or lipid peroxide (LOOH)
G6P or 6PG ... NADP+/NADPH ...
FAD/FADH2 ... GSSG/GSH ...
enz-Se/enz-SeH ... LOOH/LOH + HOH
Note that when all components are present,
this sequence functions to eliminate
lipid peroxides and excess hydrogen peroxide.
Deficiency of any components, precursors, or
supportive nutrients results in relative failure.
Supportive nutrients: carbohydrates, chromium,
niacin, riboflavin, cysteine, selenium.
The active centers of many of the component enzymes
utilize thiols (RSH), or selenols (RSeH).
These groups are susceptible to inhibition by:
lead, mercury, cadmium, arsenic.
~~~ CAUSES OF GLUTATHIONE DEPLETION ~~~
protein malnutrition
(absence of the precursors
especially cysteine)
toxic heavy metals
(inhibition by ligand binding
to glutathione)
xenobiotic overload
(detoxification by conjugation
with glutathione)
~~~ GLUTATHIONE - S - TRANSFERASE ~~~
This enzyme attaches of one molecule
of GSH to each molecule of xenobiotic.
GSH + X ---> GS-XH
Glutamic acid and glycine are removed.
GS-XH ---> cys-XH + glu + gly
Next an acetyl group is attached
to the amino group of cysteine.
CH3CO-enz + cys-XH --->
CH3CO-cys-XH + H-enz
The product is a "mercapturic acid" or
N-acetyl-L-cysteine-S-xenobiotic.
Thus xenobiotics deplete cysteine and so
inhibit other glutathione dependent functions.
~~~ CONSEQUENCES OF GLUTATHIONE DEPLETION ~~~
intracellular oxyradical sensitivity
extracellular quenching failure
peroxide toxicity
aldehyde intolerance
xenobiotic intolerance
enhanced heavy metal toxicity
immune dysregulation
~~~ TESTS FOR GLUTATHIONE DEPLETION ~~~
Urinary Mercapturates
Urinary Sulfate
Hair Sulfur
Amino Acid Analysis
(Especially Cysteine Level)
RBC Glutathione
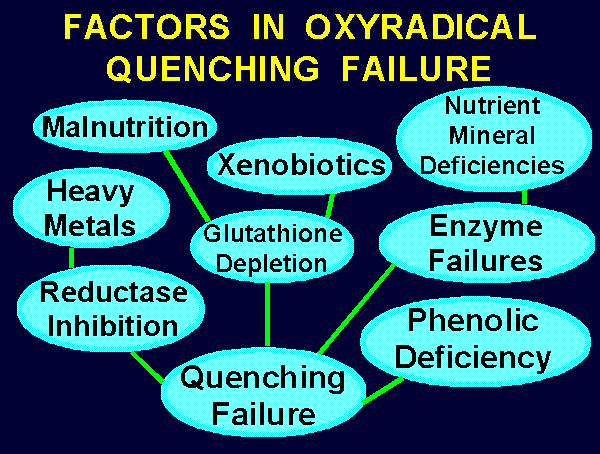
~~~ FACTORS IN OXYRADICAL QUENCHING FAILURE ~~~
Toxic Heavy Metals ---> Reductase Inhibition
Malnutrition ---> Glutathione Insufficiency
Xenobiotic Overload ---> Glutathione Depletion
Selenium Deficiency ---> Glutathione Peroxidase Insuff.
Manganese Deficiency ---> Mitochondrial SOD Insuff.
Copper or Zinc Deficiency ---> Cytoplasmic SOD Insuff.
Phenolic Deficiency ---> Reductant Insufficiency
Ascorbic Acid Deficiency ---> Reductant Insuff.
~~~ MONO-OXYGENASES GENERAL MECHANISM ~~~
These enzymes add one atom of oxygen
to their respective substrates.
Diatomic oxygen (O2) is the oxidant source.
Two reducing equivalents are required to convert
one of the oxygen atoms to water,
while the other is added to the substrate.
The overall reaction occurs as follows:
X + OO + 2[H] ---> XO + HOH
~~~ EXAMPLES OF REACTIONS CATALYSED BY MONO-OXYGENASES ~~~
PHENYLALANINE-4-MONOOXYGENASE (using biopterin):
Phe + OO + BH4 ---> Tyr + H2O + BH2
FLAVIN LINKED MONOOXYGENASE (as in bacteria):
X + OO + FADH2 ---> XO + H2O + FAD
CYSTEINE DIOXYGENASE (note 2 step addition of oxygen):
Cys-SH + OO + NADH + H+ ---> Cys-SOH + H2O + NAD+
Cys-SOH + OO + NADH + H+ ---> Cys-SO2H + H2O + NAD+
MIXED FUNCTION OXIDASES (using cytochrome P450):
NADPH...FAD/FADH2...Fe2S2-ox/Fe2S2-red...CP-Fe+++/CP-Fe++
X + OO + 2(CP450-Fe++) + 2H+ ---> XO + H2O + 2(CP450-Fe+++)
FATTY ACID DESATURASES (using cytochrome B5):
NADPH ... FAD/FADH2 ... CytB5-Fe+++/CytB5-Fe++
LH2 + OO + 2(CytB5-Fe++) + 2H+
---> L + 2H2O + 2(CytB5-Fe+++)
~~~ OXIDATIVE ELIMINATION OF:
ALDEHYDES, SULFITES, and XANTHINES ~~~
The process utilizes: water, molybdenum, flavin,
iron, and oxygen.
The substrate is hydrated by the addition of water.
X + H2O ---> HXOH
The enzyme dehydrogenates the hydrated substrate.
HXOH + enz ---> XO + enzH2
This process can be summarized by the following BETS.
XO/HXOH ... Mo(+6)/Mo(+5) ... FAD/FADH* ...
Fe+++/Fe++ ... O2/-OO*
~~~ OXIDATIVE DEGRADATION AND DETOXIFICATION ~~~
HYDROCARBONS such as the following are oxidized
by specialized enzymatic mechanisms:
carbohydrates, fats, steroids,
carboxylic acids, amino acids.
Many of the steps in these degradation processes
involve biological electron transfers sequences.
In many cases NADH or NADPH are also generated.
PRIMARY AMINES (R-CH2-NH2) are eliminated by oxidation.
Examples: amino acids, neurotransmitters,
vasopressors, histamine,
polyamines, toxic amines, etc.
Numerous amine oxidases exist which utilize
copper, FAD, or TPQ in their active centers
and catalyze the following reaction:
R-CH2-NH2 + enz ---> R-CH=NH + enzH2
The imine produced by such reactions is unstable and
readily hydrolyses to produce an aldehyde and ammonia.
R-CH=NH + H2O ---> R-CH=O + NH3
~~~ POLYAMINES AND THEIR OXIDATION PRODUCTS ~~~
PUTRESCINE:
NH2-CH2-CH2-CH2-CH2-NH2
1 2 3 4
CADAVERINE:
NH2-CH2-CH2-CH2-CH2-CH2-NH2
1 2 3 4 5
SPERMIDINE:
NH2-CH2-CH2-CH2-NH-CH2-CH2-CH2-CH2-NH2
1 2 3 1 2 3 4
SPERMINE:
NH2-CH2-CH2-CH2-NH-CH2-CH2-CH2-CH2-NH-CH2-CH2-CH2-NH2
1 2 3 1 2 3 4 1 2 3
AMINO-ALDEHYDES:
NH2--CH2-- ... --CH2--CHO
DIALDEHYDES:
CHO--CH2-- ... --CH2--CHO
HOME~~~PREV~~~NEXT


