Would you like to make this site your homepage? It's fast and easy...
Yes, Please make this my home page!
HOME~~~PREV~~~NEXT
~~~ CHEMICAL BOND TYPES ~~~
Several types of chemical bonds exist which hold
atoms together within and between molecules.
IONIC BONDS
ION DIPOLE ATTRACTIONS
DIPOLE-DIPOLE ATTRACTIONS
LIGAND-CATION COMPLEXES
CHELATE CATION COMPLEXES
HYDROGEN BONDS
METALLIC BONDS
COVALENT BONDS
~~~ COVALENT BOND TYPES ~~~
In covalent bonds two electrons (each of opposite
magnetic polarity or "spin") are paired and shared.

BOND TYPES SYMBOLS:
Sigma X--X
Sigma & Pi X==X
Conjugate X==X--X==X

~~~ BOUND RADICALS ~~~
BOUND RADICALS are parts of a molecule which are
covalently bound to the rest of the molecule.
They are often thought of as rays or limbs.
Examples:
methyl groups as in DMSO and dimethyl glycine
O CH3 O
|| | ||
CH3--S--CH3 CH3--N--CH2--C--OH
hydroxyl groups as in alcohols and phenols
HC==CH
CH3--CH2--OH / \
HC C--OH
\\ //
HC--CH
amino groups as in urea and lysine
O HO--C==O
|| |
NH2--C--NH2 NH2--CH2--CH2--CH2--CH2--CH--NH2
~~~ FREE RADICALS ~~~
= "FREE RADICALS" are molecular species possessing
an unpaired electron.
= Neither is this unpaired electron shared
between two atoms as in a covalent bond.
= Free radicals may be thought of as limbs or rays
which were broken away from a covalent molecule.
= The unpaired electron functions like a tiny magnet
seeking to be paired with another electron
of opposite magnetic polarity or "spin".
= Examples of carbon centered free radicals:
H H H O
"ethyl" HC--C* "acetyl" HC--C*
H H H
HC==CH HC==CH
/ \ / \
"aryl" HC C* "pyridinyl" RN HC*
\\ // \ /
HC--CH HC==CH
 ~~~ RESONANCE ~~~
Conjugated pi bonds enable negative charges,
positive charges, and unpaired electrons
to shift positions or resonate.
Resonance delocalizes the effects of the
characteristics being shifted.
Resonance makes conjugated products
more energy favorable.
Resonance allows for reactivity to shift
to each permitted position.
*
X--X==X--X==X--X==X--X==X
*
X==X--X--X==X--X==X--X==X
*
X==X--X==X--X--X==X--X==X
*
X==X--X==X--X==X--X--X==X
*
X==X--X==X--X==X--X==X--X
~~~ REACTIONS OF CARBON CENTERED FREE RADICALS ~~~
COUPLING:
R* + R'* ---> R--R'
REDUCTION:
R* + AOH ---> RH + AO*
OXIDATION:
H H H H
R--C--C* + [O] ---> C==C + H[O]*
H H R H
ADDITION OF DIATOMIC OXYGEN:
R* + O2 ---> ROO*
ADDUCT TO PI BOND:
R* + X==X ---> R--X--X*
~~~ OXYGEN CENTERED FREE RADICALS ~~~
These are generally recognized as dangerous.
hydroxyl = HO* hydroperoxyl = HOO*
alkoxyl = RO* alkylperoxyl = ROO*
~~~ REACTIONS OF OXYGEN CENTERED FREE RADICALS ~~~
HYDROGEN ABSTRACTION:
RO* + AOH ---> ROH + AO*
RO* + LH ---> ROH + L*
INITIATION OF LIPID PEROXIDATION:
RO* + LH ---> ROH + L*
L* + O2 ---> LOO*
ADDUCT FORMATION:
RO* + X==X ---> RO--X--X*
INITIATION OF POLYMERIZATION:
RO* + X==X ---> RO--X--X*
RO--X--X* + Y==Y ---> RO--X--X--Y--Y*
~~~ LIPID PEROXIDES ARE PRODUCED ~~~
BY FREE RADICAL CHAIN REACTIONS
HCH HCH HCH
/ \ / \ / \ + R* --->
HC==CH HC==CH
HCH HC* HCH
/ \ / \ / \ + RH
HC==CH HC==CH
1) INITIATION: R* + LH --> RH + L*
2) PROPAGATION: L* + O2 --> LOO*
LOO* + LH --> LOOH + L*
3) TERMINATION: LOO* + L* --> LOOL
L* + L* --> LL
LOO* + AOH --> LOOH + AO*
4) ELIMINATION: LOOH + 2[H] --> LOH + H2O
~~~ HOW CONJUGATED DIENES ARE PRODUCED ~~~
1. An initiator or propagator abstracts hydrogen from an
allylic carbon situated between two double bonds:
H *
--C==C--C--C==C-- + ROO* ---> --C==C--C--C==C-- + ROOH
H H H H H H H H H H
2. The adjacent pi bonds permit the unpaired electron to
shift positions and thus be delocalized among 3 sites:
* * *
--C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C--
H H H H H H H H H H H H H H H
(left) (middle) (right)
3. Diatomic oxygen can couple with the carbon centered
radical at any one of these three sites:
O* O* O*
O O O
--C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C--
H H H H H H H H H H H H H H H
4. Reduction to LOOH and subsequently to LOH yeilds
alpha hydroxy conjugated dienes 2 out of 3 times.
OH OH OH
O O O
--C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C--
H H H H H H H H H H H H H H H
~~~ ALDEHYDES PRODUCED BY SUBSEQUENT BREAKDOWN OF LOOH ~~~
These products are proinflammatory and cytotoxic.
MALONDIALDEHYDE (MDA):
H H H
O==C--C--C==O
H
4-HYDROXY-2,3-TRANS-NONENAL (4HNE):
H H H H H H H H
HC-C-C-C-C-C-C=C-C=O
H H H H H | H
OH
~~~ OXYRADICAL PRODUCTION MECHANISMS ~~~
THERMAL CLEAVAGE OF PEROXIDES:
HOOH + heat ---> HO* + HO*
ROOH + heat ---> RO* + HO*
UNIVALENT REDUCTION OF PEROXIDES:
HOOH + Fe++ ---> HO* + OH- + Fe+++
ROOH + Fe++ ---> RO* + OH- + Fe+++
ADDITION OF DIATOMIC OXYGEN TO ALKYL RADICALS:
R--CH2* + O2 ---> R--CH2--O--O*
UNIVALENT REDUCTION OF DIATOMIC OXYGEN:
Fe++ + O2 + H+ ---> Fe+++ + HOO*
*QH or *FADH + O2 ---> Q or FAD + HOO*
RADIATION:
hv + X--X ---> X* + e- + X+
hv + HOH ---> HO* + e- + H+
hv + X==X ---> *X--X*
ISCHEMIA - REPERFUSION (let [EC] = electron carriers):
[EC] + e- ---> [EC]e-
[EC]e- + O2 ---> [EC] + *OO-
~~~ PHYSIOLOGIC GENERATION OF OXYRADICALS ~~~
Macrophages, natural killer cells, and other activated
leukocytes generate superoxide from NADPH or NADH.
NADPH + (H+) + 2*OO* ---> 2(H+) + 2-OO* + NAD+
Some superoxide becomes its more dangerous conjugate acid.
(H+) + -OO* ---> HOO*
Hydroperoxyl radical is further reduced by ascorbate.
2HOO* + HOC=COH ---> 2HOOH + O=C-C=O
Hypochlorite can then be produced by myeloperoxidase.
Cl- + HOOH ---> ClO- + HOH
Superoxide or hydrogen peroxide can be oxidized
to become singlet oxygen.
-OO* + [O] ---> O=O + [O]-
HOOH + HCl0 ---> O=O + HOH + Cl- + H+
~~~ NITRIC OXIDE AS OXIDANT DEFENSE SUBSTANCE ~~~
The enzyme "nitric oxide synthase" (NOS) uses
NADPH and arginine to produce nitric oxide (*NO).
Some of the superoxide (-OO*) produced physiologically
couples with nitric oxide to produce peroxynitric acid,
a powerful cytotoxic agent towards pathogens and tumors.
H+ + -OO* + *NO ---> HOONO
Peroxynitric acid can readily decompose to release
hydroxyl radical and nitrogen dioxide.
HOONO ---> HO* + *NO2
~~~ PROOXIDANT ASSAULT WEAPONS OF KILLER CELLS ~~~
Superoxide -OO*
Singlet Oxygen O=O
Hydroperoxyl HOO*
Hydrogen Peroxide HOOH
Hypochlorous Acid HClO
Nitric Oxide *NO
Peroxynitric Acid HOONO
Hydroxyl HO*
~~~ HYDROXYL RADICAL PRODUCTION SEQUENCES ~~~
Hydroxyl radicals can be produced by Fenton's reagent.
HOOH + Fe++ ---> OH- + HO* + Fe+++
Superoxide can serve to reduce Fe+++ to Fe++,
thus enabling repetition of the Fenton reaction.
-OO* + Fe+++ <---> O2 + Fe++
Numerous other reductants including ascorbic acid and
N-acetyl-L-cysteine can reduce Fe+++ to Fe++,
thus enabling repetition of the Fenton reaction.
NALC-SH + Fe+++ ---> NALC-S* + H+ + Fe++
Thus freely diffusible cationic iron is a dangerous
initiator of toxic oxyradical activity.
~~~ HOW ASCORBIC ACID OR OTHER ENEDIOLS PLUS IRON
PRODUCE BOTH HYDROGEN PEROXIDE AND HYDROXYL RADICALS ~~~
Ascorbic acid readily reduces ferric cations to ferrous.
AAH2 + Fe+++ ---> *AAH + H+ + Fe++
Ferrous cation reversibly produces superoxide.
O2 + Fe++ <---> -OO* + Fe+++
Superoxide can be further reduced by ascorbic acid,
or it can dismutate.
-OO* + AAH2 + H+ ---> HOOH + *AAH
2 -OO* + 2 H+ ---> 2 HOOH
Ferrous cation will react with the newly formed H2O2
as in the Fenton reaction.
HOOH + Fe++ ---> OH- + HO* + Fe+++
Deferoxamine is superior to EDTA as an inhibitor
of this process.
~~~ WHY ARE FREE RADICALS BAD ? ~~~
Because the following can occur:
* peroxidize and modify lipids
* crosslink molecules
* produce allergenic adducts
* deactivate enzymes
* consume nutrients
* mutate genes
* initiate carcinogenesis
* destroy organelles
* destroy cells
* induce inflammation
* cause sclerosis
~~~ DISEASES ASSOCIATED WITH FREE RADICAL STRESS ~~~
* Iron Toxicity
* Heavy Metal Toxicity
* Arthritis
* Diabetes Mellitus
* Carcinogenesis
* Chemical Intolerance
* Macular Degeneration
* Arteriosclerosis
* Neurodegeneration
~~~ TESTS FOR OXYRADICAL STRESS ~~~
* total lipid peroxides
* aldehydes (especially malondialdehyde)
* thiobarbituric acid reactive substances (TBARS)
* conjugated dienes
* breath alkanes
* hydroxylated salicylate
* oxidatively modified cholesterols
* oxidatively modified nucleotides (esp. guanine)
* serum ascorbate/dehydroascorbate ratio
* RBC intracellular GSH/GSSG ratio
* RBC fragility
* platelet aggregation
* glycosylated proteins and AGES
* methionine sulfoxide
~~~ FACTORS IN LIPID PEROXIDATION ~~~
Iron Excess --->
Oxidases ---> Lipid <--- Hyperlipidemia
Glycosylation ---> Peroxidation
Radiation --->
Inflammation --->
~~~ RESONANCE ~~~
Conjugated pi bonds enable negative charges,
positive charges, and unpaired electrons
to shift positions or resonate.
Resonance delocalizes the effects of the
characteristics being shifted.
Resonance makes conjugated products
more energy favorable.
Resonance allows for reactivity to shift
to each permitted position.
*
X--X==X--X==X--X==X--X==X
*
X==X--X--X==X--X==X--X==X
*
X==X--X==X--X--X==X--X==X
*
X==X--X==X--X==X--X--X==X
*
X==X--X==X--X==X--X==X--X
~~~ REACTIONS OF CARBON CENTERED FREE RADICALS ~~~
COUPLING:
R* + R'* ---> R--R'
REDUCTION:
R* + AOH ---> RH + AO*
OXIDATION:
H H H H
R--C--C* + [O] ---> C==C + H[O]*
H H R H
ADDITION OF DIATOMIC OXYGEN:
R* + O2 ---> ROO*
ADDUCT TO PI BOND:
R* + X==X ---> R--X--X*
~~~ OXYGEN CENTERED FREE RADICALS ~~~
These are generally recognized as dangerous.
hydroxyl = HO* hydroperoxyl = HOO*
alkoxyl = RO* alkylperoxyl = ROO*
~~~ REACTIONS OF OXYGEN CENTERED FREE RADICALS ~~~
HYDROGEN ABSTRACTION:
RO* + AOH ---> ROH + AO*
RO* + LH ---> ROH + L*
INITIATION OF LIPID PEROXIDATION:
RO* + LH ---> ROH + L*
L* + O2 ---> LOO*
ADDUCT FORMATION:
RO* + X==X ---> RO--X--X*
INITIATION OF POLYMERIZATION:
RO* + X==X ---> RO--X--X*
RO--X--X* + Y==Y ---> RO--X--X--Y--Y*
~~~ LIPID PEROXIDES ARE PRODUCED ~~~
BY FREE RADICAL CHAIN REACTIONS
HCH HCH HCH
/ \ / \ / \ + R* --->
HC==CH HC==CH
HCH HC* HCH
/ \ / \ / \ + RH
HC==CH HC==CH
1) INITIATION: R* + LH --> RH + L*
2) PROPAGATION: L* + O2 --> LOO*
LOO* + LH --> LOOH + L*
3) TERMINATION: LOO* + L* --> LOOL
L* + L* --> LL
LOO* + AOH --> LOOH + AO*
4) ELIMINATION: LOOH + 2[H] --> LOH + H2O
~~~ HOW CONJUGATED DIENES ARE PRODUCED ~~~
1. An initiator or propagator abstracts hydrogen from an
allylic carbon situated between two double bonds:
H *
--C==C--C--C==C-- + ROO* ---> --C==C--C--C==C-- + ROOH
H H H H H H H H H H
2. The adjacent pi bonds permit the unpaired electron to
shift positions and thus be delocalized among 3 sites:
* * *
--C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C--
H H H H H H H H H H H H H H H
(left) (middle) (right)
3. Diatomic oxygen can couple with the carbon centered
radical at any one of these three sites:
O* O* O*
O O O
--C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C--
H H H H H H H H H H H H H H H
4. Reduction to LOOH and subsequently to LOH yeilds
alpha hydroxy conjugated dienes 2 out of 3 times.
OH OH OH
O O O
--C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C--
H H H H H H H H H H H H H H H
~~~ ALDEHYDES PRODUCED BY SUBSEQUENT BREAKDOWN OF LOOH ~~~
These products are proinflammatory and cytotoxic.
MALONDIALDEHYDE (MDA):
H H H
O==C--C--C==O
H
4-HYDROXY-2,3-TRANS-NONENAL (4HNE):
H H H H H H H H
HC-C-C-C-C-C-C=C-C=O
H H H H H | H
OH
~~~ OXYRADICAL PRODUCTION MECHANISMS ~~~
THERMAL CLEAVAGE OF PEROXIDES:
HOOH + heat ---> HO* + HO*
ROOH + heat ---> RO* + HO*
UNIVALENT REDUCTION OF PEROXIDES:
HOOH + Fe++ ---> HO* + OH- + Fe+++
ROOH + Fe++ ---> RO* + OH- + Fe+++
ADDITION OF DIATOMIC OXYGEN TO ALKYL RADICALS:
R--CH2* + O2 ---> R--CH2--O--O*
UNIVALENT REDUCTION OF DIATOMIC OXYGEN:
Fe++ + O2 + H+ ---> Fe+++ + HOO*
*QH or *FADH + O2 ---> Q or FAD + HOO*
RADIATION:
hv + X--X ---> X* + e- + X+
hv + HOH ---> HO* + e- + H+
hv + X==X ---> *X--X*
ISCHEMIA - REPERFUSION (let [EC] = electron carriers):
[EC] + e- ---> [EC]e-
[EC]e- + O2 ---> [EC] + *OO-
~~~ PHYSIOLOGIC GENERATION OF OXYRADICALS ~~~
Macrophages, natural killer cells, and other activated
leukocytes generate superoxide from NADPH or NADH.
NADPH + (H+) + 2*OO* ---> 2(H+) + 2-OO* + NAD+
Some superoxide becomes its more dangerous conjugate acid.
(H+) + -OO* ---> HOO*
Hydroperoxyl radical is further reduced by ascorbate.
2HOO* + HOC=COH ---> 2HOOH + O=C-C=O
Hypochlorite can then be produced by myeloperoxidase.
Cl- + HOOH ---> ClO- + HOH
Superoxide or hydrogen peroxide can be oxidized
to become singlet oxygen.
-OO* + [O] ---> O=O + [O]-
HOOH + HCl0 ---> O=O + HOH + Cl- + H+
~~~ NITRIC OXIDE AS OXIDANT DEFENSE SUBSTANCE ~~~
The enzyme "nitric oxide synthase" (NOS) uses
NADPH and arginine to produce nitric oxide (*NO).
Some of the superoxide (-OO*) produced physiologically
couples with nitric oxide to produce peroxynitric acid,
a powerful cytotoxic agent towards pathogens and tumors.
H+ + -OO* + *NO ---> HOONO
Peroxynitric acid can readily decompose to release
hydroxyl radical and nitrogen dioxide.
HOONO ---> HO* + *NO2
~~~ PROOXIDANT ASSAULT WEAPONS OF KILLER CELLS ~~~
Superoxide -OO*
Singlet Oxygen O=O
Hydroperoxyl HOO*
Hydrogen Peroxide HOOH
Hypochlorous Acid HClO
Nitric Oxide *NO
Peroxynitric Acid HOONO
Hydroxyl HO*
~~~ HYDROXYL RADICAL PRODUCTION SEQUENCES ~~~
Hydroxyl radicals can be produced by Fenton's reagent.
HOOH + Fe++ ---> OH- + HO* + Fe+++
Superoxide can serve to reduce Fe+++ to Fe++,
thus enabling repetition of the Fenton reaction.
-OO* + Fe+++ <---> O2 + Fe++
Numerous other reductants including ascorbic acid and
N-acetyl-L-cysteine can reduce Fe+++ to Fe++,
thus enabling repetition of the Fenton reaction.
NALC-SH + Fe+++ ---> NALC-S* + H+ + Fe++
Thus freely diffusible cationic iron is a dangerous
initiator of toxic oxyradical activity.
~~~ HOW ASCORBIC ACID OR OTHER ENEDIOLS PLUS IRON
PRODUCE BOTH HYDROGEN PEROXIDE AND HYDROXYL RADICALS ~~~
Ascorbic acid readily reduces ferric cations to ferrous.
AAH2 + Fe+++ ---> *AAH + H+ + Fe++
Ferrous cation reversibly produces superoxide.
O2 + Fe++ <---> -OO* + Fe+++
Superoxide can be further reduced by ascorbic acid,
or it can dismutate.
-OO* + AAH2 + H+ ---> HOOH + *AAH
2 -OO* + 2 H+ ---> 2 HOOH
Ferrous cation will react with the newly formed H2O2
as in the Fenton reaction.
HOOH + Fe++ ---> OH- + HO* + Fe+++
Deferoxamine is superior to EDTA as an inhibitor
of this process.
~~~ WHY ARE FREE RADICALS BAD ? ~~~
Because the following can occur:
* peroxidize and modify lipids
* crosslink molecules
* produce allergenic adducts
* deactivate enzymes
* consume nutrients
* mutate genes
* initiate carcinogenesis
* destroy organelles
* destroy cells
* induce inflammation
* cause sclerosis
~~~ DISEASES ASSOCIATED WITH FREE RADICAL STRESS ~~~
* Iron Toxicity
* Heavy Metal Toxicity
* Arthritis
* Diabetes Mellitus
* Carcinogenesis
* Chemical Intolerance
* Macular Degeneration
* Arteriosclerosis
* Neurodegeneration
~~~ TESTS FOR OXYRADICAL STRESS ~~~
* total lipid peroxides
* aldehydes (especially malondialdehyde)
* thiobarbituric acid reactive substances (TBARS)
* conjugated dienes
* breath alkanes
* hydroxylated salicylate
* oxidatively modified cholesterols
* oxidatively modified nucleotides (esp. guanine)
* serum ascorbate/dehydroascorbate ratio
* RBC intracellular GSH/GSSG ratio
* RBC fragility
* platelet aggregation
* glycosylated proteins and AGES
* methionine sulfoxide
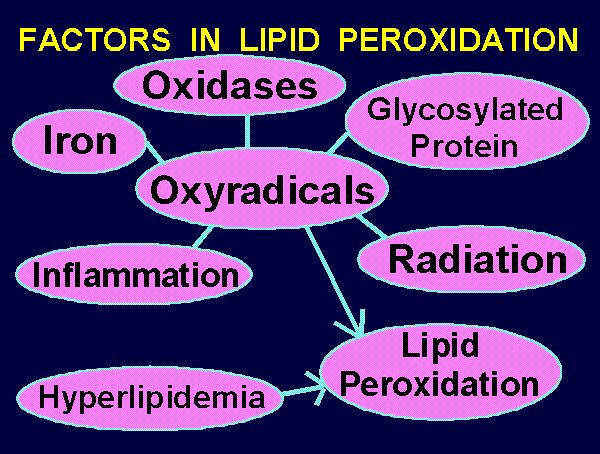
~~~ FACTORS IN LIPID PEROXIDATION ~~~
Iron Excess --->
Oxidases ---> Lipid <--- Hyperlipidemia
Glycosylation ---> Peroxidation
Radiation --->
Inflammation --->
~~~ ATHEROGENS ~~~
Atherogenic Products Of Oxyradical
Induced Modifications Of Lipids:
* lipid peroxides
* aldehydes
* oxycholesterols
* lysolecithins
~~~ OXYCHOLESTEROLS ~~~
These are especially irritating and atherogenic
forms of oxidatively modified cholesterol.
They are produced by oxyradicals acting
within LDL particles and within atheromas.
* 7-beta-hydroperoxycholesterol
* 7-beta-hydroxycholesterol
* 7-ketocholesterol
* 5-alpha-epoxycholesterol
* 6-alpha-epoxycholesterol
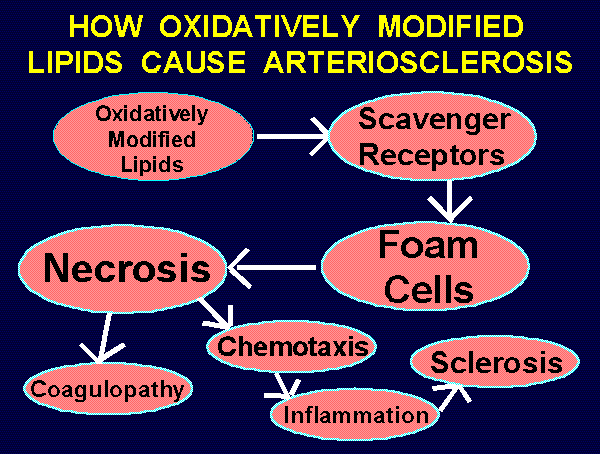
~~~ HOW OXIDATIVELY MODIFIED LIPIDS CAUSE ARTERIOSCLEROSIS ~~~
Oxidatively modified lipids --->
Uptake by scavenger receptors --->
Conversion of macrophages to "foam cells" --->
Necrosis of foam cells --->
Coagulopathy & Chemotaxis & Chronic inflammation --->
Sclerosis
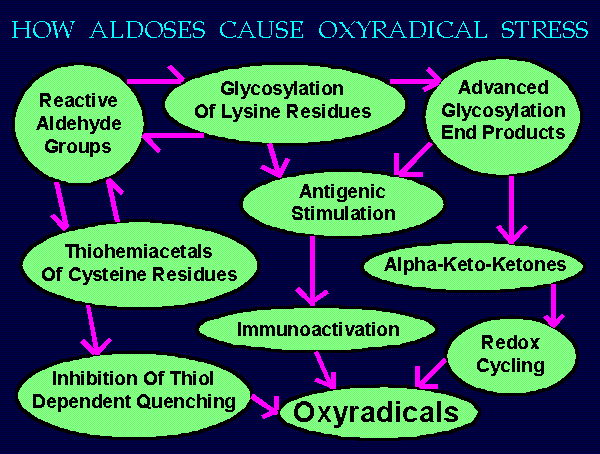
~~~ HOW ALDOSES CAUSE OXYRADICAL STRESS ~~~
Sugars exist as both opened and closed chain isomers.
When open, an aldehyde group is available for reactions.
During episodes of hyperglycemia as in diabetes, there
are proportionately elevated levels of open forms.
These react as listed below causing oxyradical stress.
Reactive Aldehyde Groups ---> Thiohemiacetals --->
Inhibition Of Quenching By Thiols --->
Increased Oxyradicals
Reactive Aldehyde Groups --->
Glycosylated Lysine Residues --->
Advanced Glycosylated End Products (AGES) --->
Antigenic Stimulation ---> Immunoactivation --->
Production Of Various Oxyradicals
AGES ---> Alpha-keto-ketones --->
Redox Cycling ---> Superoxide & Hydrogen Peroxide
~~~ NONENZYMATIC OXYRADICAL QUENCHING MECHANISMS ~~~
Coupling:
HO* + HO* ---> HOOH
RO* + R'O* ---> ROOR'
ROO* + R'* ---> ROOR'
RS* + RS'* ---> RSSR'
Addition Of Oxygen Followed By Reduction:
R* + O2 ---> ROO*
ROO* + AOH ---> ROOH + AO*
Addition Of Proton Followed By Reduction:
-OO* + H+ ---> HOO*
HOO* + AOH ---> HOOH + AO*
Spontaneous Dismutation Of Superoxide:
2 -OO* + 2 H+ ---> O2 + HOOH
Trapping By Pi Bonds:
HO* + X==X ---> HO--X--X*
Reduction by Alcohols, Enols, Enediols,
Monophenols, Diphenols, and Polyphenols:
ROO* + R'OH ---> ROOH + RO*
Reduction by Thiols:
RO* + R'SH ---> ROH + R'S*
Reduction by Amines:
HO* + RNH2 ---> HOH + RHN*
~~~ PREVENTION OF OXYRADICAL OVERPRODUCTION ~~~
- Radiation Avoidance
- Xenobiotic Avoidance
- Glycemic Control
- Inflammation Control
- Optimal Nutrition:
B-complex, amino acids, trace minerals, cofactors
- Supplementation of Quenchers:
carotenoids, thiols, enediols, phenols
- Enzymatic Reduction of Peroxides
by Glutathione Peroxidase:
N-acetyl-L-cysteine, selenium
- Chelation of Transition Metals
- Chelation of Toxic Heavy Metals
HOME~~~PREV~~~NEXT
~~~ RESONANCE ~~~ Conjugated pi bonds enable negative charges, positive charges, and unpaired electrons to shift positions or resonate. Resonance delocalizes the effects of the characteristics being shifted. Resonance makes conjugated products more energy favorable. Resonance allows for reactivity to shift to each permitted position. * X--X==X--X==X--X==X--X==X * X==X--X--X==X--X==X--X==X * X==X--X==X--X--X==X--X==X * X==X--X==X--X==X--X--X==X * X==X--X==X--X==X--X==X--X ~~~ REACTIONS OF CARBON CENTERED FREE RADICALS ~~~ COUPLING: R* + R'* ---> R--R' REDUCTION: R* + AOH ---> RH + AO* OXIDATION: H H H H R--C--C* + [O] ---> C==C + H[O]* H H R H ADDITION OF DIATOMIC OXYGEN: R* + O2 ---> ROO* ADDUCT TO PI BOND: R* + X==X ---> R--X--X* ~~~ OXYGEN CENTERED FREE RADICALS ~~~ These are generally recognized as dangerous. hydroxyl = HO* hydroperoxyl = HOO* alkoxyl = RO* alkylperoxyl = ROO* ~~~ REACTIONS OF OXYGEN CENTERED FREE RADICALS ~~~ HYDROGEN ABSTRACTION: RO* + AOH ---> ROH + AO* RO* + LH ---> ROH + L* INITIATION OF LIPID PEROXIDATION: RO* + LH ---> ROH + L* L* + O2 ---> LOO* ADDUCT FORMATION: RO* + X==X ---> RO--X--X* INITIATION OF POLYMERIZATION: RO* + X==X ---> RO--X--X* RO--X--X* + Y==Y ---> RO--X--X--Y--Y* ~~~ LIPID PEROXIDES ARE PRODUCED ~~~ BY FREE RADICAL CHAIN REACTIONS HCH HCH HCH / \ / \ / \ + R* ---> HC==CH HC==CH HCH HC* HCH / \ / \ / \ + RH HC==CH HC==CH 1) INITIATION: R* + LH --> RH + L* 2) PROPAGATION: L* + O2 --> LOO* LOO* + LH --> LOOH + L* 3) TERMINATION: LOO* + L* --> LOOL L* + L* --> LL LOO* + AOH --> LOOH + AO* 4) ELIMINATION: LOOH + 2[H] --> LOH + H2O ~~~ HOW CONJUGATED DIENES ARE PRODUCED ~~~ 1. An initiator or propagator abstracts hydrogen from an allylic carbon situated between two double bonds: H * --C==C--C--C==C-- + ROO* ---> --C==C--C--C==C-- + ROOH H H H H H H H H H H 2. The adjacent pi bonds permit the unpaired electron to shift positions and thus be delocalized among 3 sites: * * * --C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C-- H H H H H H H H H H H H H H H (left) (middle) (right) 3. Diatomic oxygen can couple with the carbon centered radical at any one of these three sites: O* O* O* O O O --C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C-- H H H H H H H H H H H H H H H 4. Reduction to LOOH and subsequently to LOH yeilds alpha hydroxy conjugated dienes 2 out of 3 times. OH OH OH O O O --C--C==C--C==C-- --C==C--C--C==C-- --C==C--C==C--C-- H H H H H H H H H H H H H H H ~~~ ALDEHYDES PRODUCED BY SUBSEQUENT BREAKDOWN OF LOOH ~~~ These products are proinflammatory and cytotoxic. MALONDIALDEHYDE (MDA): H H H O==C--C--C==O H 4-HYDROXY-2,3-TRANS-NONENAL (4HNE): H H H H H H H H HC-C-C-C-C-C-C=C-C=O H H H H H | H OH ~~~ OXYRADICAL PRODUCTION MECHANISMS ~~~ THERMAL CLEAVAGE OF PEROXIDES: HOOH + heat ---> HO* + HO* ROOH + heat ---> RO* + HO* UNIVALENT REDUCTION OF PEROXIDES: HOOH + Fe++ ---> HO* + OH- + Fe+++ ROOH + Fe++ ---> RO* + OH- + Fe+++ ADDITION OF DIATOMIC OXYGEN TO ALKYL RADICALS: R--CH2* + O2 ---> R--CH2--O--O* UNIVALENT REDUCTION OF DIATOMIC OXYGEN: Fe++ + O2 + H+ ---> Fe+++ + HOO* *QH or *FADH + O2 ---> Q or FAD + HOO* RADIATION: hv + X--X ---> X* + e- + X+ hv + HOH ---> HO* + e- + H+ hv + X==X ---> *X--X* ISCHEMIA - REPERFUSION (let [EC] = electron carriers): [EC] + e- ---> [EC]e- [EC]e- + O2 ---> [EC] + *OO- ~~~ PHYSIOLOGIC GENERATION OF OXYRADICALS ~~~ Macrophages, natural killer cells, and other activated leukocytes generate superoxide from NADPH or NADH. NADPH + (H+) + 2*OO* ---> 2(H+) + 2-OO* + NAD+ Some superoxide becomes its more dangerous conjugate acid. (H+) + -OO* ---> HOO* Hydroperoxyl radical is further reduced by ascorbate. 2HOO* + HOC=COH ---> 2HOOH + O=C-C=O Hypochlorite can then be produced by myeloperoxidase. Cl- + HOOH ---> ClO- + HOH Superoxide or hydrogen peroxide can be oxidized to become singlet oxygen. -OO* + [O] ---> O=O + [O]- HOOH + HCl0 ---> O=O + HOH + Cl- + H+ ~~~ NITRIC OXIDE AS OXIDANT DEFENSE SUBSTANCE ~~~ The enzyme "nitric oxide synthase" (NOS) uses NADPH and arginine to produce nitric oxide (*NO). Some of the superoxide (-OO*) produced physiologically couples with nitric oxide to produce peroxynitric acid, a powerful cytotoxic agent towards pathogens and tumors. H+ + -OO* + *NO ---> HOONO Peroxynitric acid can readily decompose to release hydroxyl radical and nitrogen dioxide. HOONO ---> HO* + *NO2 ~~~ PROOXIDANT ASSAULT WEAPONS OF KILLER CELLS ~~~ Superoxide -OO* Singlet Oxygen O=O Hydroperoxyl HOO* Hydrogen Peroxide HOOH Hypochlorous Acid HClO Nitric Oxide *NO Peroxynitric Acid HOONO Hydroxyl HO* ~~~ HYDROXYL RADICAL PRODUCTION SEQUENCES ~~~ Hydroxyl radicals can be produced by Fenton's reagent. HOOH + Fe++ ---> OH- + HO* + Fe+++ Superoxide can serve to reduce Fe+++ to Fe++, thus enabling repetition of the Fenton reaction. -OO* + Fe+++ <---> O2 + Fe++ Numerous other reductants including ascorbic acid and N-acetyl-L-cysteine can reduce Fe+++ to Fe++, thus enabling repetition of the Fenton reaction. NALC-SH + Fe+++ ---> NALC-S* + H+ + Fe++ Thus freely diffusible cationic iron is a dangerous initiator of toxic oxyradical activity. ~~~ HOW ASCORBIC ACID OR OTHER ENEDIOLS PLUS IRON PRODUCE BOTH HYDROGEN PEROXIDE AND HYDROXYL RADICALS ~~~ Ascorbic acid readily reduces ferric cations to ferrous. AAH2 + Fe+++ ---> *AAH + H+ + Fe++ Ferrous cation reversibly produces superoxide. O2 + Fe++ <---> -OO* + Fe+++ Superoxide can be further reduced by ascorbic acid, or it can dismutate. -OO* + AAH2 + H+ ---> HOOH + *AAH 2 -OO* + 2 H+ ---> 2 HOOH Ferrous cation will react with the newly formed H2O2 as in the Fenton reaction. HOOH + Fe++ ---> OH- + HO* + Fe+++ Deferoxamine is superior to EDTA as an inhibitor of this process. ~~~ WHY ARE FREE RADICALS BAD ? ~~~ Because the following can occur: * peroxidize and modify lipids * crosslink molecules * produce allergenic adducts * deactivate enzymes * consume nutrients * mutate genes * initiate carcinogenesis * destroy organelles * destroy cells * induce inflammation * cause sclerosis ~~~ DISEASES ASSOCIATED WITH FREE RADICAL STRESS ~~~ * Iron Toxicity * Heavy Metal Toxicity * Arthritis * Diabetes Mellitus * Carcinogenesis * Chemical Intolerance * Macular Degeneration * Arteriosclerosis * Neurodegeneration ~~~ TESTS FOR OXYRADICAL STRESS ~~~ * total lipid peroxides * aldehydes (especially malondialdehyde) * thiobarbituric acid reactive substances (TBARS) * conjugated dienes * breath alkanes * hydroxylated salicylate * oxidatively modified cholesterols * oxidatively modified nucleotides (esp. guanine) * serum ascorbate/dehydroascorbate ratio * RBC intracellular GSH/GSSG ratio * RBC fragility * platelet aggregation * glycosylated proteins and AGES * methionine sulfoxide ~~~ FACTORS IN LIPID PEROXIDATION ~~~ Iron Excess ---> Oxidases ---> Lipid <--- Hyperlipidemia Glycosylation ---> Peroxidation Radiation ---> Inflammation --->