|
|
|
|
On The Mechanisms Of Toxicity Of Chlorine Oxides Against Malarial Parasites - An Overview |
|---|
 can be acidified as a convenient method to produce
chlorine dioxide (ClO2)
can be acidified as a convenient method to produce
chlorine dioxide (ClO2)
 which is a
strong oxidant and a potent disinfectant. A protocol has
been developed whereby a solution of these compounds can
be taken orally. This procedure rapidly eliminates malaria
and other infectious agents in only one dose. Chlorine
dioxide (ClO2) is highly reactive with thiols (RSH)
which is a
strong oxidant and a potent disinfectant. A protocol has
been developed whereby a solution of these compounds can
be taken orally. This procedure rapidly eliminates malaria
and other infectious agents in only one dose. Chlorine
dioxide (ClO2) is highly reactive with thiols (RSH)
 , polyamines
, polyamines
 , purines
, purines
 , certain amino acids and iron, all of which are
necessary for the growth and survival of pathogenic
microbes. Properly dosed this new treatment is tolerable
orally with only mild or transient side effects.
More research to better document efficacy in malaria
and in other infections is urgently called for.
, certain amino acids and iron, all of which are
necessary for the growth and survival of pathogenic
microbes. Properly dosed this new treatment is tolerable
orally with only mild or transient side effects.
More research to better document efficacy in malaria
and in other infections is urgently called for.
 is prepared. The remaining 20% is a mixture of the usual
excipients necessary in the manufacture and stabilization
of sodium chlorite (NaClO2) powder or flake. Such are mostly sodium chloride (NaCl) ~19%
is prepared. The remaining 20% is a mixture of the usual
excipients necessary in the manufacture and stabilization
of sodium chlorite (NaClO2) powder or flake. Such are mostly sodium chloride (NaCl) ~19%
 , sodium hydroxide (NaOH) <1%
, sodium hydroxide (NaOH) <1%
 , and sodium chlorate (NaClO3) <1%
, and sodium chlorate (NaClO3) <1%
 . The actual sodium chlorite (NaClO2) present is
therefore 22.4%. Using a medium caliber dropper (25 drops
per cc), the usual administered dose per treatment is 6 to
15 drops. In terms of milligrams of sodium chlorite (NaClO2), this
calculates out to 9mg per drop or 54mg to 135mg per treatment.
Effectiveness is enhanced, if prior to administration
the selected drops are premixed with a nontoxic
nonreducing strong acid and allowed to react for 3 minutes.
Examples include: acetic acid (vinegar)
. The actual sodium chlorite (NaClO2) present is
therefore 22.4%. Using a medium caliber dropper (25 drops
per cc), the usual administered dose per treatment is 6 to
15 drops. In terms of milligrams of sodium chlorite (NaClO2), this
calculates out to 9mg per drop or 54mg to 135mg per treatment.
Effectiveness is enhanced, if prior to administration
the selected drops are premixed with a nontoxic
nonreducing strong acid and allowed to react for 3 minutes.
Examples include: acetic acid (vinegar)
 , citric acid,
, citric acid,  lactic acid, phosphoric acid, or hydrochloric acid.
The acids neutralize the sodium hydroxide (NaOH)
lactic acid, phosphoric acid, or hydrochloric acid.
The acids neutralize the sodium hydroxide (NaOH)
 and at the same time convert the chlorite (ClO2-)
and at the same time convert the chlorite (ClO2-)
 to its conjugate acid known as chlorous acid (HClO2).
to its conjugate acid known as chlorous acid (HClO2).
 Next this chlorous acid (HClO2)
Next this chlorous acid (HClO2)
 oxidizes adjacent chlorite anions (ClO2-)
oxidizes adjacent chlorite anions (ClO2-)
 removing one electron from each. This converts each effected chlorite molecule
(ClO2-)
removing one electron from each. This converts each effected chlorite molecule
(ClO2-)  to become chlorine dioxide (ClO2).
to become chlorine dioxide (ClO2).
 Chlorine dioxide (ClO2) appears in solution as a yellow tint
which smells exactly like elemental chlorine (Cl2)
Chlorine dioxide (ClO2) appears in solution as a yellow tint
which smells exactly like elemental chlorine (Cl2)
 .
The resultant solution must always be diluted
by mixing this into a glass of water (preferably chilled)
before being taken orally (preferably on an empty stomach).
The above described drink can be repeated
a few hours later if clinically deemed necessary.
Considerably lower dosing should be applied in children or
in emaciated individuals scaled down according to size or weight.
The diluted solution can be taken without food to enhance effectiveness
but this often causes nausea. Drinking extra water (especially cold water)
usually relieves this nausea. Also nausea is less likely to occur,
if prior to treatment starchy food is present in the stomach.
Protein must never be present in the stomach as protein reacts with
and quenches chlorine dioxide (ClO2)
.
The resultant solution must always be diluted
by mixing this into a glass of water (preferably chilled)
before being taken orally (preferably on an empty stomach).
The above described drink can be repeated
a few hours later if clinically deemed necessary.
Considerably lower dosing should be applied in children or
in emaciated individuals scaled down according to size or weight.
The diluted solution can be taken without food to enhance effectiveness
but this often causes nausea. Drinking extra water (especially cold water)
usually relieves this nausea. Also nausea is less likely to occur,
if prior to treatment starchy food is present in the stomach.
Protein must never be present in the stomach as protein reacts with
and quenches chlorine dioxide (ClO2)
 .
Reducing acids such ascorbic acid (vitamin C)
.
Reducing acids such ascorbic acid (vitamin C)
 or alpha-lipoic acid (also known as thioctic acid)
or alpha-lipoic acid (also known as thioctic acid)
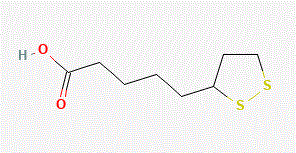 or its dihydro version
or its dihydro version
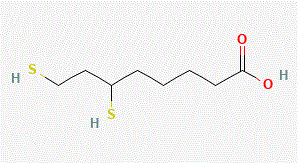 must never be present in the mixtures or in the stomach at the time of treatment.
These will quench the chlorine dioxide (ClO2) rendering it ineffective.
For the same reason antioxidant supplements should not be taken on the day of treatment.
Other side effects reported (though considerably less common)
are transient vomiting, diarrhea, headache, dizziness, lethargy or malaise.
must never be present in the mixtures or in the stomach at the time of treatment.
These will quench the chlorine dioxide (ClO2) rendering it ineffective.
For the same reason antioxidant supplements should not be taken on the day of treatment.
Other side effects reported (though considerably less common)
are transient vomiting, diarrhea, headache, dizziness, lethargy or malaise.
 and chlorine dioxide (ClO2)
and chlorine dioxide (ClO2)
 are already widely used as disinfectants.
What is novel and exciting here is that Mr. Humble's technique
seems: 1) easy to use, 2) rapidly acting, 3) successful,
4) apparently lacking in toxicity, and 5) affordable.
If this treatment continues to prove effective, it could be
used to help rid the world of one of the most devasting of all
known plagues. Especially moving in me is the empathy I feel
for anyone with a debilitating febrile illness. I cannot forget
how horrible I feel whenever I have caught influenza. How much
more miserable it must be to suffer like that again and again
every 2 to 3 days as happens in malaria. Millions of people
suffer this way year round. 1 to 3 million die from malaria
every year mostly children. Thus motivated I sought to learn
all I could about the chemistry of the oxides of chlorine.
I wanted to understand their probable mechanisms of toxicity
towards the causative agents of malaria (Plasmodium species).
I wanted to check available literature pertaining to issues
of safety or risk in human use.
are already widely used as disinfectants.
What is novel and exciting here is that Mr. Humble's technique
seems: 1) easy to use, 2) rapidly acting, 3) successful,
4) apparently lacking in toxicity, and 5) affordable.
If this treatment continues to prove effective, it could be
used to help rid the world of one of the most devasting of all
known plagues. Especially moving in me is the empathy I feel
for anyone with a debilitating febrile illness. I cannot forget
how horrible I feel whenever I have caught influenza. How much
more miserable it must be to suffer like that again and again
every 2 to 3 days as happens in malaria. Millions of people
suffer this way year round. 1 to 3 million die from malaria
every year mostly children. Thus motivated I sought to learn
all I could about the chemistry of the oxides of chlorine.
I wanted to understand their probable mechanisms of toxicity
towards the causative agents of malaria (Plasmodium species).
I wanted to check available literature pertaining to issues
of safety or risk in human use.
 , zinc peroxide
, zinc peroxide
 , various quinones (e.g. benzoquinone
, various quinones (e.g. benzoquinone
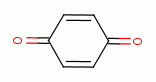 , rhodizonic acid)
, rhodizonic acid)
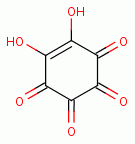 , various glyoxals (e.g. glyoxal
, various glyoxals (e.g. glyoxal
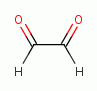 , methyl glyoxal
, methyl glyoxal
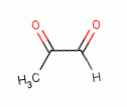 , ozone
, ozone
 , ultraviolet light, hyperbaric oxygen
, benzoyl peroxide
, ultraviolet light, hyperbaric oxygen
, benzoyl peroxide
 , anodes, artemisinin
, anodes, artemisinin
 , methylene blue
, methylene blue
 , allicin
, allicin
 ,
iodine
,
iodine
 and
permanganate
and
permanganate
 . Some work has been done using dilute solutions
of sodium chlorite (NaClO2)
. Some work has been done using dilute solutions
of sodium chlorite (NaClO2)
 internally to treat fungal infections,
chronic fatigue, and cancer; however, little has been
published in that regard.
internally to treat fungal infections,
chronic fatigue, and cancer; however, little has been
published in that regard.
Low dose oxidant exposure to living red blood cells induces an increase
in 2,3-diphosphoglycerate
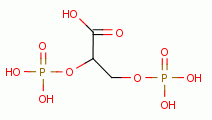 levels inside these cells. This attaches to hemoglobin (Hb) in such a way
that oxyhemoglobin (HbO2) more readily releases oxygen (O2)
levels inside these cells. This attaches to hemoglobin (Hb) in such a way
that oxyhemoglobin (HbO2) more readily releases oxygen (O2)
 to the tissues throughout the body.
to the tissues throughout the body.
Hyperbaric oxygenation (oxygen under pressure):
Taken internally, intermittently and in low doses many
oxidants have been found to be powerful immune stimulants.
Sodium chlorite (NaClO2)
 acidified with lactic acid
acidified with lactic acid
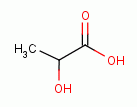 as in the product "WF10" has similarly been shown to modulate immune activation.
Exposure of live blood to ultraviolet light also has immune
enhancing effects. These treatments work through a natural
physiologic trigger mechanism, which induces peripheral white
blood cells to express and to release cytokines. These
cytokines serve as a control system to down-regulate allergic
reactions and as an alarm system to increase cellular attack
against pathogens.
as in the product "WF10" has similarly been shown to modulate immune activation.
Exposure of live blood to ultraviolet light also has immune
enhancing effects. These treatments work through a natural
physiologic trigger mechanism, which induces peripheral white
blood cells to express and to release cytokines. These
cytokines serve as a control system to down-regulate allergic
reactions and as an alarm system to increase cellular attack
against pathogens.
Activated cells of the immune system naturally produce strong
oxidants as part of the inflammatory process at sites
of infection or cancer to rid the body of these diseases.
Examples are:
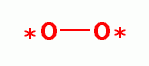
superoxide (*OO-)
 , hydrogen peroxide (H2O2)
, hydrogen peroxide (H2O2)
 , hydroxyl radical (HO*)
, hydroxyl radical (HO*)
 , singlet oxygen (O=O)
, singlet oxygen (O=O)
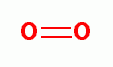 and ozone (O3)
and ozone (O3)
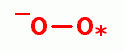
 . Another is peroxynitrate (-OONO)
. Another is peroxynitrate (-OONO)
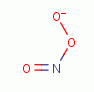 the coupled product of superoxide (*OO-)
the coupled product of superoxide (*OO-)
 and nitric oxide (*NO)
and nitric oxide (*NO)
 radicals.
radicals.
| -OO* + *NO -> -OONO |
|---|
Yet another is hypochlorous acid (HOCl)
 the conjugate acid of sodium hypochlorite (NaClO)
the conjugate acid of sodium hypochlorite (NaClO)
 . The immune system uses these oxidants
to attack various parasites.
. The immune system uses these oxidants
to attack various parasites.
 are commonly used as bleaching agents, as swimming pool sanitizers,
and as disinfectants. At low concentrations chlorine dioxide (ClO2)
are commonly used as bleaching agents, as swimming pool sanitizers,
and as disinfectants. At low concentrations chlorine dioxide (ClO2)
 has been shown to kill many types of bacteria, viruses and
protozoa. Ozone (O3)
has been shown to kill many types of bacteria, viruses and
protozoa. Ozone (O3)
 or chlorine dioxide (ClO2)
or chlorine dioxide (ClO2)
 are often used
to disinfect public water supplies or to sanitize and deodorize
waste water. Sodium chlorite (NaClO2)
are often used
to disinfect public water supplies or to sanitize and deodorize
waste water. Sodium chlorite (NaClO2)
 or chlorine dioxide (ClO2)
or chlorine dioxide (ClO2)
 solutions are used in certain mouth washes to clear mouth odors
and oral bacteria. Chlorine dioxide (ClO2) sanitizes food preparation
facilities. Acidified sodium chlorite is FDA approved as a spray
in the meat packing industry to sanitized meat. This can also
be used to sanitize vegetables and other foods. Farmers use
this to cleanse the udders of cows to prevent mastitis, or
to rid eggs of pathogenic bacteria. Chlorine dioxide (ClO2)
can be used to disinfect endoscopes. Oxidants such as iodine
solutions are used in certain mouth washes to clear mouth odors
and oral bacteria. Chlorine dioxide (ClO2) sanitizes food preparation
facilities. Acidified sodium chlorite is FDA approved as a spray
in the meat packing industry to sanitized meat. This can also
be used to sanitize vegetables and other foods. Farmers use
this to cleanse the udders of cows to prevent mastitis, or
to rid eggs of pathogenic bacteria. Chlorine dioxide (ClO2)
can be used to disinfect endoscopes. Oxidants such as iodine
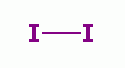 , various peroxides
, various peroxides
 , permanganate
, permanganate
 and chlorine dioxide
and chlorine dioxide
 can be applied topically to the skin to treat infections caused
by bacteria or fungi.
can be applied topically to the skin to treat infections caused
by bacteria or fungi.
 , artemether
, artemether
 , t-butyl hydroperoxide
, t-butyl hydroperoxide
 , xanthone
, xanthone
 , various quinones
(e.g. atovaquone
, various quinones
(e.g. atovaquone
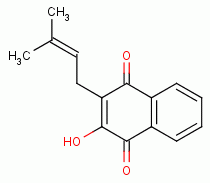
 , lapachol
, lapachol
 , beta-lapachone
, beta-lapachone
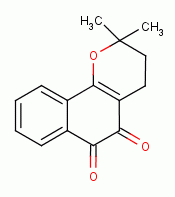 , menadione
, menadione
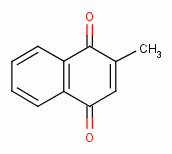 ) and methylene blue
) and methylene blue
 .
.
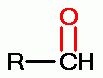
 . Thiols as a class
behave as reductants (electron donors). As such they are
especially sensitive to oxidants (electron grabbers).
. Thiols as a class
behave as reductants (electron donors). As such they are
especially sensitive to oxidants (electron grabbers). 
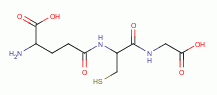 and other sulfur compounds are
reactive with sodium chlorite (NaClO2)
and other sulfur compounds are
reactive with sodium chlorite (NaClO2)
 and with chlorine dioxide (ClO2)
and with chlorine dioxide (ClO2)
 . These are the very agents present in Mr. Humble's
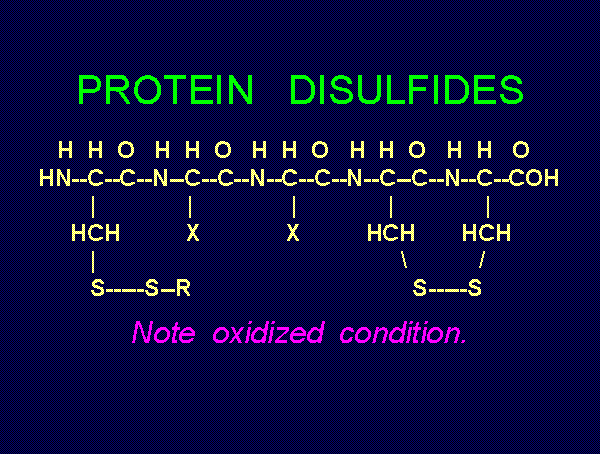
solution. Possible products of oxidation of thiols (RSH)
. These are the very agents present in Mr. Humble's
solution. Possible products of oxidation of thiols (RSH)
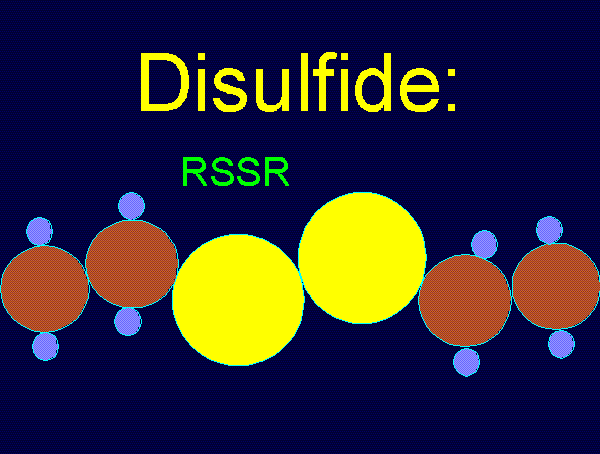
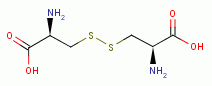
 using various oxides of chlorine are: disulfides (RSSR)
using various oxides of chlorine are: disulfides (RSSR)
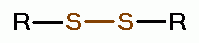 , disulfide monoxides (RSSOR)
, disulfide monoxides (RSSOR)
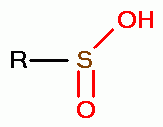
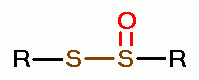 , sulfenic acids (RSOH)
, sulfenic acids (RSOH)
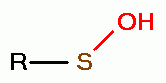 , sulfinic acids (RSO2H)
, sulfinic acids (RSO2H)
 and sulfonic acids (RSO3H)
and sulfonic acids (RSO3H)
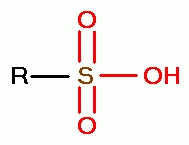 .
. 
 by oxidation, the parasite
rapidly dies. A list of thiols (RSH) upon which survival
of Plasmodium species heavily depend includes:
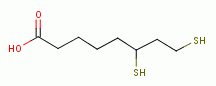
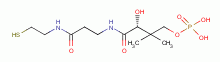
dihydrolipoic acid
by oxidation, the parasite
rapidly dies. A list of thiols (RSH) upon which survival
of Plasmodium species heavily depend includes:
dihydrolipoic acid
 , coenzyme A
, coenzyme A
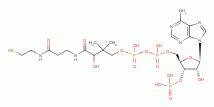 and acyl carrier protein
and acyl carrier protein
 , glutathione
, glutathione
 , glutathione reductase,
glutathione-S-transferase, peroxiredoxin, thioredoxin,
glutaredoxin, plasmoredoxin, thioredoxin reductase,
falcipain and ornithine decarboxylase.
, glutathione reductase,
glutathione-S-transferase, peroxiredoxin, thioredoxin,
glutaredoxin, plasmoredoxin, thioredoxin reductase,
falcipain and ornithine decarboxylase. 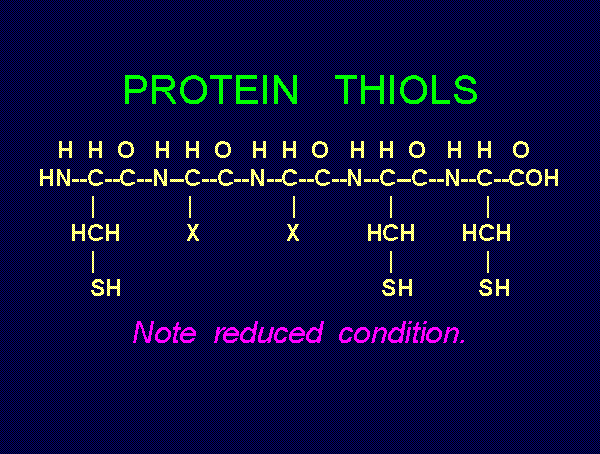
 to the more
active chlorous acid (HClO2)
to the more
active chlorous acid (HClO2)
 or chlorine dioxide (ClO2)
or chlorine dioxide (ClO2)
 right inside the parasite. Furthermore, Plasmodia consume 50 to 100
times more glucose than noninfected red blood cells most of
which is metabolized to lactic acid
right inside the parasite. Furthermore, Plasmodia consume 50 to 100
times more glucose than noninfected red blood cells most of
which is metabolized to lactic acid
 another known activator of chlorite (ClO2-).
another known activator of chlorite (ClO2-).
Next falcipain a hemoglobin digesting enzyme hydrolyzes
hemoglobin protein to release its nutritional amino acids.
A necessary byproduct of this digestion is the release of 4 heme molecules
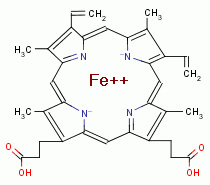 from each hemoglobin molecule digested.
Free heme (also known as ferriprotoporphyrin IX) is redox
active and can react with ambient oxygen (O2)
from each hemoglobin molecule digested.
Free heme (also known as ferriprotoporphyrin IX) is redox
active and can react with ambient oxygen (O2)
 , an abundance
of which is always present in red blood cells. This produces
superoxide radical (*OO-)
, an abundance
of which is always present in red blood cells. This produces
superoxide radical (*OO-)
 , hydrogen peroxide (H2O2)
, hydrogen peroxide (H2O2)
 and
other reactive oxidant toxic species (ROTS). These can
rapidly poison the parasite internally. To protect themselves
against this dangerous side-effect of eating blood protein,
Plasmodia must maintain a high reductant capacity
(an abundance of reduced thiols (RSH)
and
other reactive oxidant toxic species (ROTS). These can
rapidly poison the parasite internally. To protect themselves
against this dangerous side-effect of eating blood protein,
Plasmodia must maintain a high reductant capacity
(an abundance of reduced thiols (RSH)
 and NADPH
and NADPH
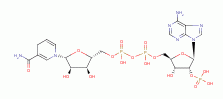 ) to quench these ROTS.
This is their main mechanism of antioxidant defense.
) to quench these ROTS.
This is their main mechanism of antioxidant defense.
Plasmodia must also rapidly and continuously eliminate heme
 ,
which is accomplished by two methods. Firstly, heme is
polymerized producing hemozoin. Secondly, heme is metabolized
in a detoxification process that requires reduced glutathione
(GSH)
,
which is accomplished by two methods. Firstly, heme is
polymerized producing hemozoin. Secondly, heme is metabolized
in a detoxification process that requires reduced glutathione
(GSH)
 . Therefore any method (especially exposure to oxidants)
which limits the availability of reduced glutathione (GSH)
will cause a toxic build up of heme and of ROTS inside the
parasite cells. Sodium chlorite (NaClO2) and chlorine dioxide (ClO2)
(the exact agents present in Mr. Humble's treatment) readily
oxidize glutathione (GSH). Therefore, a rapid killing of Plasmodia
upon taking acidified sodium chlorite orally should be
expected.
. Therefore any method (especially exposure to oxidants)
which limits the availability of reduced glutathione (GSH)
will cause a toxic build up of heme and of ROTS inside the
parasite cells. Sodium chlorite (NaClO2) and chlorine dioxide (ClO2)
(the exact agents present in Mr. Humble's treatment) readily
oxidize glutathione (GSH). Therefore, a rapid killing of Plasmodia
upon taking acidified sodium chlorite orally should be
expected.
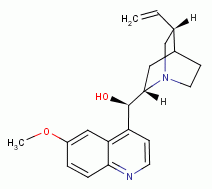 , chloroquine
, chloroquine
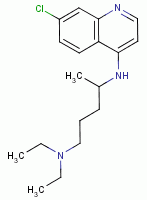 , mefloquine
, mefloquine
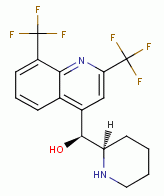 , quinacrine
, quinacrine
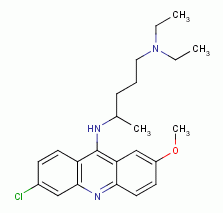 , amodiaquine
, amodiaquine
 , primaquine
, primaquine
 and other quinoline-like antibiotics
and other quinoline-like antibiotics
 all work by blocking the
heme
all work by blocking the
heme
 detoxifying system inside the trophozoites. Many
Plasmodial strains against which quinolines have repeatedly
been used have found ways to adapt to these drugs and to
acquire resistance. Research into the mechanisms of resistance
has found that often resistance is accomplished by a meere
upregulation of glutathione (GSH)
detoxifying system inside the trophozoites. Many
Plasmodial strains against which quinolines have repeatedly
been used have found ways to adapt to these drugs and to
acquire resistance. Research into the mechanisms of resistance
has found that often resistance is accomplished by a meere
upregulation of glutathione (GSH)
 production and utilization.
Consequently oxidizing or otherwise depleting glutathione (GSH)
inside the parasite usually restores sensitivity to the
quinoline antibiotics. Therefore, protocols combining the use
of oxidants with quinolines are under developement and already
showing signs of success. In this context let us consider that
no amount of intraplasmodial glutathione (GSH) could ever
resist exposure to a suffient dose of chlorine dioxide (ClO2)
production and utilization.
Consequently oxidizing or otherwise depleting glutathione (GSH)
inside the parasite usually restores sensitivity to the
quinoline antibiotics. Therefore, protocols combining the use
of oxidants with quinolines are under developement and already
showing signs of success. In this context let us consider that
no amount of intraplasmodial glutathione (GSH) could ever
resist exposure to a suffient dose of chlorine dioxide (ClO2)
 . Note that each molecule of chlorine dioxide (ClO2) can disable 1 to 5
molecules of glutathione (GSH) depending on the reaction mechanism.
. Note that each molecule of chlorine dioxide (ClO2) can disable 1 to 5
molecules of glutathione (GSH) depending on the reaction mechanism.
|
2(GSH) + 2(ClO2) --> 1(GSSG) + 2(H+) + 2(ClO2-) or 10(GSH) + 2(ClO2) --> 5(GSSG) + 2(H+) + 2(Cl-) + 4(H2O) |
|---|
 by virtue of its oxidative power. However, quinolines contain
secondary amino groups
by virtue of its oxidative power. However, quinolines contain
secondary amino groups
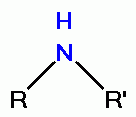 or tertiary amino groups
or tertiary amino groups
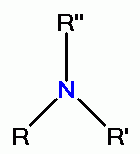 which react with chlorine dioxide (ClO2)
which react with chlorine dioxide (ClO2)
 in such a way that both could destroy each other.
Some possible strategies to resolve this incompatibility are
suggested below.
in such a way that both could destroy each other.
Some possible strategies to resolve this incompatibility are
suggested below.
 and many other drugs if they have an unoxidized sulfur atom,
a phenol group
and many other drugs if they have an unoxidized sulfur atom,
a phenol group
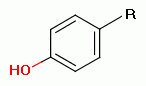 , a secondary amine
, a secondary amine
 or a tertiary amine.
or a tertiary amine.
 Such are reactive with the chlorine dioxide (ClO2)
Such are reactive with the chlorine dioxide (ClO2)
 component.
component.
 .
.
| 2 [H] + (RSSR) -> 2(RSH) |
|---|
This system is known as the hexose monophophate shunt.
A key player in this system is the enzyme glucose-6-phosphate-
dehydrogenase (G6PDH).
 This enzyme is an essential part of a complex process that
produces NADPH
This enzyme is an essential part of a complex process that
produces NADPH
 the main provider of reductants to the reductases
(enzymes which convert oxidized sulfur compounds back into thiols (RSH)
the main provider of reductants to the reductases
(enzymes which convert oxidized sulfur compounds back into thiols (RSH)
 ).
Patients with a genetic defect of G6PDH,
known as glucose-6-phosphate-dehydrogenase deficiency disease,
are especially sensitive to oxidants and to prooxidant drugs.
However, this genetic disease has a benefit in that such
individuals are naturally resistant to malaria. They can still
catch malaria, but it is much less severe in them, since they
permanently lack the enzyme necessary to assist the parasite
in reactivating glutathione disulfide (GSSG)
).
Patients with a genetic defect of G6PDH,
known as glucose-6-phosphate-dehydrogenase deficiency disease,
are especially sensitive to oxidants and to prooxidant drugs.
However, this genetic disease has a benefit in that such
individuals are naturally resistant to malaria. They can still
catch malaria, but it is much less severe in them, since they
permanently lack the enzyme necessary to assist the parasite
in reactivating glutathione disulfide (GSSG)
 and other oxidized thiols.
Chlorine dioxide (ClO2)
and other oxidized thiols.
Chlorine dioxide (ClO2)
 has been shown to oxidize and denature
G6PDH by reaction with the tyrosine
has been shown to oxidize and denature
G6PDH by reaction with the tyrosine
 and the tryptophan
and the tryptophan
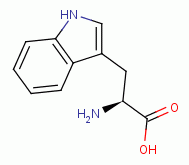 residues inside the enzyme.
Furthermore, G6PDH is sensitive to inhibition by sodium chlorate (NaClO3)
residues inside the enzyme.
Furthermore, G6PDH is sensitive to inhibition by sodium chlorate (NaClO3)
 , another member of the chlorine oxide family of compounds.
Sodium chlorate (NaClO3) is a trace
ingredient present in Jim Humble's antimalarial solution.
Some sodium chlorate (NaClO3)
, another member of the chlorine oxide family of compounds.
Sodium chlorate (NaClO3) is a trace
ingredient present in Jim Humble's antimalarial solution.
Some sodium chlorate (NaClO3)
 should also be produced in vivo by a slow reaction of chlorine
dioxide (ClO2) with water under alkaline conditions.
should also be produced in vivo by a slow reaction of chlorine
dioxide (ClO2) with water under alkaline conditions.
| 2(ClO2) + 2(OH-) -> (ClO2-) + (ClO3-) + H2O |
|---|
The Plasmodia may attempt to restore
any thiols (RSH)
 lost to oxidation. However, this becomes more
difficult as G6PDH is inhibited by chlorine dioxide (ClO2)
lost to oxidation. However, this becomes more
difficult as G6PDH is inhibited by chlorine dioxide (ClO2)
 or
by chlorate (ClO3-)
or
by chlorate (ClO3-)
 .
.
 . Other mechanisms
of toxicity of the oxides of chlorine against Plasmodia
should also be considered. Oxides of chlorine are generally
rapidly reactive with ferrous iron (Fe++) converting it to
ferric (Fe+++). This explains why in cases of overdosed
exposures to oxides of chlorine such as sodium chlorite
(NaClO2)
. Other mechanisms
of toxicity of the oxides of chlorine against Plasmodia
should also be considered. Oxides of chlorine are generally
rapidly reactive with ferrous iron (Fe++) converting it to
ferric (Fe+++). This explains why in cases of overdosed
exposures to oxides of chlorine such as sodium chlorite
(NaClO2)
 there was a notable rise in methemoglobin levels.
Methemoglobin is a metabolically inactive form of hemoglobin
in which its ferrous iron (Fe++) cofactor has been oxidized
to ferric (Fe+++).
there was a notable rise in methemoglobin levels.
Methemoglobin is a metabolically inactive form of hemoglobin
in which its ferrous iron (Fe++) cofactor has been oxidized
to ferric (Fe+++).
 In living things including parasites iron
is a necessary cofactor for many enzymes. Thus it is
reasonable to expect that any damage to Plasmodia caused
by oxides of chlorine is compounded by conversion of ferrous
(Fe++) cofactors to ferric (Fe+++) or other alterations of
iron compounds. Superoxide dismutase (SOD) inside Plasmodial
cells also utilizes iron in its active center. Chlorine dioxide
In living things including parasites iron
is a necessary cofactor for many enzymes. Thus it is
reasonable to expect that any damage to Plasmodia caused
by oxides of chlorine is compounded by conversion of ferrous
(Fe++) cofactors to ferric (Fe+++) or other alterations of
iron compounds. Superoxide dismutase (SOD) inside Plasmodial
cells also utilizes iron in its active center. Chlorine dioxide
 also oxidizes manganese (Mn++).
also oxidizes manganese (Mn++).
 , which are
deadly to parasites and to tumors. Chlorine dioxide (ClO2)
, which are
deadly to parasites and to tumors. Chlorine dioxide (ClO2)
 is known to be especially reactive against secondary amines
is known to be especially reactive against secondary amines
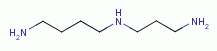
 . This includes spermine
. This includes spermine
 and spermidine
and spermidine
 the two main
biologically important polyamines.
the two main
biologically important polyamines.  and polyamines does quadruple damage to the pathogen:
and polyamines does quadruple damage to the pathogen: 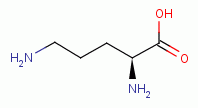
 and spermine
and spermine

 .
. 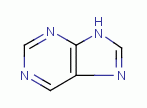 are essential to many life processes. These molecules
have a double ring structure. The rings are heterocyclic
being composed of both carbon and nitrogen. The secondary amino nitrogen atoms
are essential to many life processes. These molecules
have a double ring structure. The rings are heterocyclic
being composed of both carbon and nitrogen. The secondary amino nitrogen atoms
 are vulnerable to reaction with chlorine dioxide (ClO2)
are vulnerable to reaction with chlorine dioxide (ClO2)
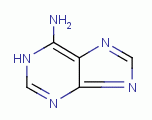
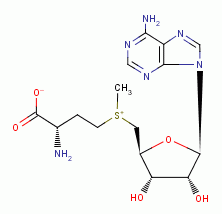
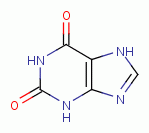
 . Examples of important biologic purines are xanthine
. Examples of important biologic purines are xanthine
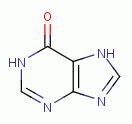
 , hypoxanthine
, hypoxanthine
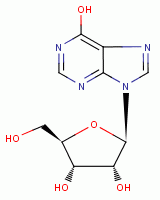
 , inosine
, inosine
 , guanine
, guanine
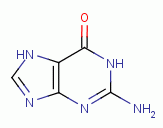 and
adenine
and
adenine
 . Guanine and adenine are
essential components of DNA and RNA necessary for all genetic
functions and for all protein syntheses. Adenine is an
essential component of the cofactors NADH
. Guanine and adenine are
essential components of DNA and RNA necessary for all genetic
functions and for all protein syntheses. Adenine is an
essential component of the cofactors NADH
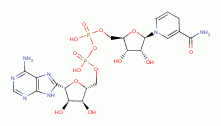 , NADPH
, NADPH
 , FAD
, FAD
 and ATP
and ATP
 , necessary for many metabolic functions including oxidation-
reduction and energy metabolism. Any purines lost because of
chlorine dioxide (ClO2)
, necessary for many metabolic functions including oxidation-
reduction and energy metabolism. Any purines lost because of
chlorine dioxide (ClO2)
 exposure can be readily replaced by host cells.
Plasmodia and other apicomplexae are uniquely vulnerable to purine
exposure can be readily replaced by host cells.
Plasmodia and other apicomplexae are uniquely vulnerable to purine
 deficiency as they lack the enzymes necessary to produce
purines for themselves. Instead these must be scavenged from
host cells and imported across the plasma membranes of the
parasite cells. Drugs are under development to inhibit purine
utilization by Plasmodia and are already showing signs of
success. Temporarily destroying some of the purines in the
blood as should occur upon brief exposure to chlorine dioxide (ClO2)
deficiency as they lack the enzymes necessary to produce
purines for themselves. Instead these must be scavenged from
host cells and imported across the plasma membranes of the
parasite cells. Drugs are under development to inhibit purine
utilization by Plasmodia and are already showing signs of
success. Temporarily destroying some of the purines in the
blood as should occur upon brief exposure to chlorine dioxide (ClO2)
 in vivo is probably an additional stress that Plasmodia cannot
tolerate.
in vivo is probably an additional stress that Plasmodia cannot
tolerate.
 is highly reactive with thiols (RSH)
is highly reactive with thiols (RSH)
 , phenols
, phenols
 , secondary amines
, secondary amines
 and tertiary amines
and tertiary amines
 . Therefore,
proteins composed of amino acids which present these
reactive groups are vulnerable to oxidation by this agent.
Proteins which present thiol groups as residue(s) of the
amino acid L-cysteine
. Therefore,
proteins composed of amino acids which present these
reactive groups are vulnerable to oxidation by this agent.
Proteins which present thiol groups as residue(s) of the
amino acid L-cysteine
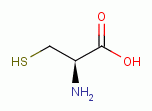 are discussed above under TARGETING THIOLS.
L-tyrosine
are discussed above under TARGETING THIOLS.
L-tyrosine
 presents a phenol group and is therefore
similarly vulnerable. L-tryptophan
presents a phenol group and is therefore
similarly vulnerable. L-tryptophan
 , L-histidine
, L-histidine
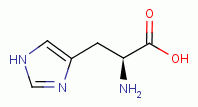 , L-proline
, L-proline
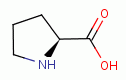 and 4-hydroy-L-proline
and 4-hydroy-L-proline
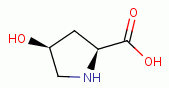 present secondary amino groups which are also
especially reactive with chlorine dioxide (ClO2)
present secondary amino groups which are also
especially reactive with chlorine dioxide (ClO2)
 .
.  levels in frequent users. Sodium chlorite (NaClO2)
levels in frequent users. Sodium chlorite (NaClO2)
 , as found in municipal water supplies after disinfection
by chorine dioxide (ClO2)
, as found in municipal water supplies after disinfection
by chorine dioxide (ClO2)
 , has been studied and proven safe.
Animal studies using much higher oral or topical doses have
proven relatively safe. In a suicide attempt 10g of sodium
chlorite (NaClO2) taken orally caused refractory methemoglobinemia
and nearly fatal kidney failure. Inhalation or aerosol exposure to
chlorine dioxide (ClO2) gas is highly irritating and generally not
recommended. Special precautions must be employed
in cases of glucose-6-phosphate-dehydrogenase deficiency
disease, as these patients are especially sensitive to oxidants
of all kinds. Nevertheless, oral acidified sodium chlorite
solutions might even be found safe and effective in them,
but probably will need to be administered at lower doses.
, has been studied and proven safe.
Animal studies using much higher oral or topical doses have
proven relatively safe. In a suicide attempt 10g of sodium
chlorite (NaClO2) taken orally caused refractory methemoglobinemia
and nearly fatal kidney failure. Inhalation or aerosol exposure to
chlorine dioxide (ClO2) gas is highly irritating and generally not
recommended. Special precautions must be employed
in cases of glucose-6-phosphate-dehydrogenase deficiency
disease, as these patients are especially sensitive to oxidants
of all kinds. Nevertheless, oral acidified sodium chlorite
solutions might even be found safe and effective in them,
but probably will need to be administered at lower doses.
 dependent parasites should also be susceptible to acidified sodium chlorite.
For example Trypanosoma and Leishmania extensively utilize and
cannot survive without the cofactor known as trypanothione.
dependent parasites should also be susceptible to acidified sodium chlorite.
For example Trypanosoma and Leishmania extensively utilize and
cannot survive without the cofactor known as trypanothione.
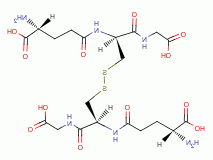
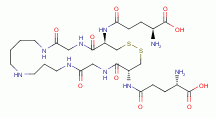 Each molecule of trypanothione presents 2 sulfur atoms and 5 secondary
amino groups all of which are vulnerable to oxidative destruction
from chlorine dioxide (ClO2)
Each molecule of trypanothione presents 2 sulfur atoms and 5 secondary
amino groups all of which are vulnerable to oxidative destruction
from chlorine dioxide (ClO2)
 .
.
Chlorine dioxide (ClO2) has been proven to be cidal to almost all known infectious agents in vitro using remarkably low concentrations. This includes parasites, fungi, bacteria and viruses. The experiences noted above imply that this compound is tolerable orally at effective concentrations. Therefore extensive research is warranted to determine if acidified sodium chlorite is effective in treating other infections. We may be on the verge of discovering the most potent and broad spectrum antimicrobial agent yet known. Special thanks go to Jim Humble for his willingness to share his discovery with the world.